|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110592 |
|---|
|
Identification |
|---|
| Name: |
α-D-mannose 1-phosphate |
|---|
| Description: | An organophosphate oxoanion that is the dianion of α-D-mannose 1-phosphate. |
|---|
|
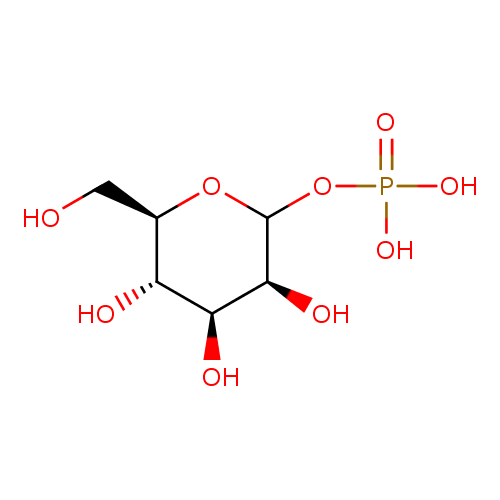
Structure |
|
|---|
| Synonyms: | -
mannose-1-phosphate
-
D-mannose-1-phosphate
-
mannose-1-P
|
|---|
|
Chemical Formula: |
C6H11O9P
|
|---|
| Average Molecular Weight: |
258.12 |
|---|
| Monoisotopic Molecular
Weight: |
260.0297185262 |
|---|
| InChI Key: |
HXXFSFRBOHSIMQ-RWOPYEJCSA-L |
|---|
| InChI: |
InChI=1S/C6H13O9P/c7-1-2-3(8)4(9)5(10)6(14-2)15-16(11,12)13/h2-10H,1H2,(H2,11,12,13)/p-2/t2-,3-,4+,5+,6-/m1/s1 |
|---|
| CAS
number: |
27251-84-9 |
|---|
| IUPAC Name: | 1-O-phosphonato-α-D-mannopyranose |
|---|
|
Traditional IUPAC Name: |
D-mannose 1-phosphate |
|---|
| SMILES: | C(O)C1(OC(OP(=O)([O-])[O-])C(O)C(O)C(O)1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as monosaccharide phosphates. These are monosaccharides comprising a phosphated group linked to the carbohydrate unit. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Monosaccharide phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hexose monosaccharide
- Monosaccharide phosphate
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- Oxane
- Alkyl phosphate
- Phosphoric acid ester
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Alcohol
- Hydrocarbon derivative
- Primary alcohol
- Organic oxide
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Fructose and Mannose Degradation pae00051
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Korner C, Lehle L, von Figura K: Abnormal synthesis of mannose 1-phosphate derived carbohydrates in carbohydrate-deficient glycoprotein syndrome type I fibroblasts with phosphomannomutase deficiency. Glycobiology. 1998 Feb;8(2):165-71. [9451026 ]
- Van Schaftingen E, Jaeken J: Phosphomannomutase deficiency is a cause of carbohydrate-deficient glycoprotein syndrome type I. FEBS Lett. 1995 Dec 27;377(3):318-20. [8549746 ]
- Orvisky E, Stubblefield B, Long RT, Martin BM, Sidransky E, Krasnewich D: Phosphomannomutase activity in congenital disorders of glycosylation type Ia determined by direct analysis of the interconversion of mannose-1-phosphate to mannose-6-phosphate by high-pH anion-exchange chromatography with pulsed amperometric detection. Anal Biochem. 2003 Jun 1;317(1):12-8. [12729595 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|