|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110583 |
|---|
|
Identification |
|---|
| Name: |
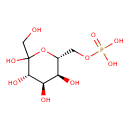
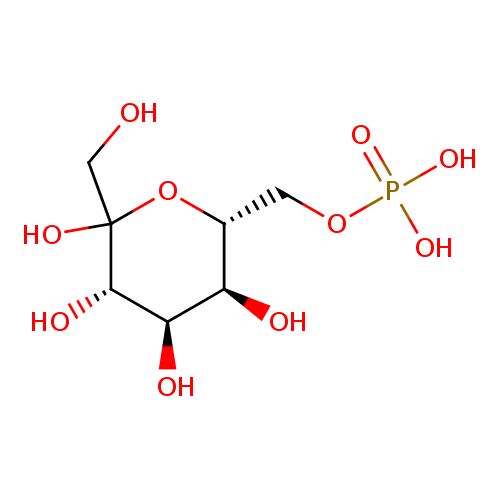
D-sedoheptulose 7-phosphate |
|---|
| Description: | An organophosphate oxoanion that is the dianion of sedoheptulose 7-phosphate arising from deprotonation of both OH groups from the phosphate. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
D-Sedoheptulose-7-P
-
heptulose 7-phosphate
-
sedoheptulose-7-P
-
sedo-heptulose-7-phosphate
|
|---|
|
Chemical Formula: |
C7H13O10P
|
|---|
| Average Molecular Weight: |
288.15 |
|---|
| Monoisotopic Molecular
Weight: |
290.0402832125 |
|---|
| InChI Key: |
JDTUMPKOJBQPKX-GBNDHIKLSA-L |
|---|
| InChI: |
InChI=1S/C7H15O10P/c8-1-3(9)5(11)7(13)6(12)4(10)2-17-18(14,15)16/h4-8,10-13H,1-2H2,(H2,14,15,16)/p-2/t4-,5-,6-,7+/m1/s1 |
|---|
| CAS
number: |
2646-35-7 |
|---|
| IUPAC Name: | 7-O-phosphonato-D-altro-hept-2-ulose |
|---|
|
Traditional IUPAC Name: |
[(2R,3S,4R,5S)-3,4,5,6-tetrahydroxy-6-(hydroxymethyl)oxan-2-yl]methoxyphosphonic acid |
|---|
| SMILES: | C(O)C(C(O)C(C(O)C(COP(=O)([O-])[O-])O)O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as hexose phosphates. These are carbohydrate derivatives containing a hexose substituted by one or more phosphate groups. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Hexose phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hexose phosphate
- C-glycosyl compound
- Glycosyl compound
- Monosaccharide phosphate
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- Oxane
- Phosphoric acid ester
- Alkyl phosphate
- Hemiacetal
- Secondary alcohol
- Organoheterocyclic compound
- Oxacycle
- Polyol
- Organic oxide
- Alcohol
- Primary alcohol
- Hydrocarbon derivative
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Nakayama Y, Kinoshita A, Tomita M: Dynamic simulation of red blood cell metabolism and its application to the analysis of a pathological condition. Theor Biol Med Model. 2005 May 9;2(1):18. [15882454 ]
- Wamelink MM, Struys EA, Huck JH, Roos B, van der Knaap MS, Jakobs C, Verhoeven NM: Quantification of sugar phosphate intermediates of the pentose phosphate pathway by LC-MS/MS: application to two new inherited defects of metabolism. J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Aug 25;823(1):18-25. Epub 2005 Jan 23. [16055050 ]
- Huck JH, Struys EA, Verhoeven NM, Jakobs C, van der Knaap MS: Profiling of pentose phosphate pathway intermediates in blood spots by tandem mass spectrometry: application to transaldolase deficiency. Clin Chem. 2003 Aug;49(8):1375-80. [12881455 ]
- Thornalley PJ, Jahan I, Ng R: Suppression of the accumulation of triosephosphates and increased formation of methylglyoxal in human red blood cells during hyperglycaemia by thiamine in vitro. J Biochem (Tokyo). 2001 Apr;129(4):543-9. [11275553 ]
- Makarov SA, Kudriavtseva GV, Kolotilova AI: [Effect of prostaglandins F2 and F2 alpha on the pentosephosate pathway in human blood platelets] Vopr Med Khim. 1983 Sep-Oct;29(5):27-32. [6316661 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|