|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110582 |
|---|
|
Identification |
|---|
| Name: |
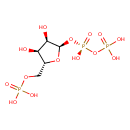
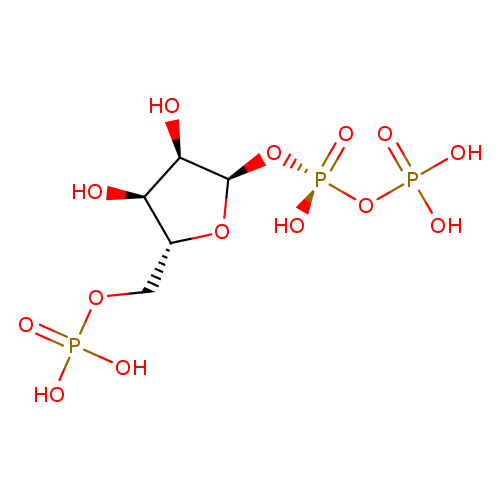
5-phospho-α-D-ribose 1-diphosphate |
|---|
| Description: | Pentaanion of 5-O-phosphono-α-D-ribofuranosyl diphosphate arising from deprotonation of the phosphate and diphosphate OH groups; major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
PRPP
-
5-phosphoribosyl 1-pyrophosphate
-
5-phosphoribosyl diphosphate
-
5-phosphoribosyl-1-PP
-
5-phosphoribosyl-PP
-
5-phosphoribosyl-1-pyrophosphate
-
5-phosphoribosylpyrophosphate
-
phosphoribosylpyrophosphate
-
5-phospho-ribosyl-pyrophosphate
-
α-D-5-phosphoribosylPP
-
α-D-5-P-RibosylPP
|
|---|
|
Chemical Formula: |
C5H8O14P3
|
|---|
| Average Molecular Weight: |
385.03 |
|---|
| Monoisotopic Molecular
Weight: |
389.9518146567 |
|---|
| InChI Key: |
PQGCEDQWHSBAJP-TXICZTDVSA-I |
|---|
| InChI: |
InChI=1S/C5H13O14P3/c6-3-2(1-16-20(8,9)10)17-5(4(3)7)18-22(14,15)19-21(11,12)13/h2-7H,1H2,(H,14,15)(H2,8,9,10)(H2,11,12,13)/p-5/t2-,3-,4-,5-/m1/s1 |
|---|
| CAS
number: |
7540-64-9 |
|---|
| IUPAC Name: | [({[(2R,3R,4S,5R)-3,4-dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl]oxy}(hydroxy)phosphoryl)oxy]phosphonic acid |
|---|
|
Traditional IUPAC Name: |
phosphoribosylpyrophosphate |
|---|
| SMILES: | C(OP(=O)([O-])[O-])C1(OC(OP([O-])(=O)OP([O-])(=O)[O-])C(O)C(O)1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pentose phosphates. These are carbohydrate derivatives containing a pentose substituted by one or more phosphate groups. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Pentose phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pentose phosphate
- Pentose-5-phosphate
- Monosaccharide phosphate
- Organic pyrophosphate
- Monoalkyl phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Oxolane
- Secondary alcohol
- 1,2-diol
- Organoheterocyclic compound
- Oxacycle
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
- 5-O-phosphono-D-ribofuranosyl diphosphate (CHEBI:17111 )
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -5 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Snyder FF, Dyer C, Seegmiller JE, Goldblum RM, Mills GC, Schmalstieg FC: Substrate inhibition of adenosine phosphorylation in adenosine deaminase deficiency and adenosine-mediated inhibition of PP-ribose-P dependent nucleotide synthesis in hypoxanthine phosphoribosyltransferase deficient erythrocytes. J Inherit Metab Dis. 1988;11(2):174-83. [2459496 ]
- Gordon RB, Keough DT, Emmerson BT: HPRT-deficiency associated with normal PRPP concentration and APRT activity. J Inherit Metab Dis. 1987;10(1):82-8. [2437388 ]
- Nishida Y, Akaoka I, Nishizawa T, Maruki M, Maruki K: Synthesis and concentration of 5-phosphoribosyl-1-pyrophosphate in erythrocytes from patients with Down's syndrome. Ann Rheum Dis. 1977 Jun;36(3):261-3. [141914 ]
- Ghitis J, Schreiber C, Waxman S: Phosphate-induced phosphoribosylpyrophosphate elevations to assess deranged folate and purine nucleotide metabolism. Proc Soc Exp Biol Med. 1987 Oct;186(1):90-5. [2442765 ]
- Yamaoka T, Yano M, Kondo M, Sasaki H, Hino S, Katashima R, Moritani M, Itakura M: Feedback inhibition of amidophosphoribosyltransferase regulates the rate of cell growth via purine nucleotide, DNA, and protein syntheses. J Biol Chem. 2001 Jun 15;276(24):21285-91. Epub 2001 Apr 4. [11290738 ]
- Blinov MN, Kamyshentsev MV, Luganova IS, Filanovskaia LI, Filippova VN: [Phosphoribosyl pyrophosphate and its metabolic enzymes in the erythrocytes in certain forms of anemia] Vopr Med Khim. 1976 Jul-Aug;22(4):456-62. [194412 ]
- Micheli V, Taddeo A: [Spectrophotometric assay of 5-phosphoribosyl-1-pyrophosphate synthetase (PRPP) in erythrocyte lysate (author's transl)] Quad Sclavo Diagn. 1981 Jun;17(2):209-15. [6267652 ]
- Sakuma R, Nishina T, Yamanaka H, Kamatani N, Nishioka K, Maeda M, Tsuji A: Phosphoribosylpyrophosphate synthetase in human erythrocytes: assay and kinetic studies using high-performance liquid chromatography. Clin Chim Acta. 1991 Dec 16;203(2-3):143-52. [1663846 ]
- Zoref-Shani E, Feinstein S, Frishberg Y, Bromberg Y, Sperling O: Kelley-Seegmiller syndrome due to a unique variant of hypoxanthine-guanine phosphoribosyltransferase: reduced affinity for 5-phosphoribosyl-1-pyrophosphate manifested only at low, physiological substrate concentrations. Biochim Biophys Acta. 2000 Feb 21;1500(2):197-203. [10657589 ]
- Sperling O, Boer P, Brosh S, Elazar E, Szeinberg A, de Vries A: Normal activity of metabolic pathways involved in the formation and utilization of phosphoribosylpyrophosphate in erythrocytes of patients with primary metabolic gout. Nutr Metab. 1975;18(4):217-23. [172821 ]
- Gorbach ZV: [Determination of phosphoribosyl pyrophosphate in the erythrocytes] Lab Delo. 1977;(12):724-5. [75318 ]
- Marcolongo R, Pompucci G, Micheli V: Familial distribution of increased erythrocyte PP-ribose-P levels. Adv Exp Med Biol. 1977;76A:280-6. [193371 ]
- Becker MA, Losman MJ, Itkin P, Simkin PA: Gout with superactive phosphoribosylpyrophosphate synthetase due to increased enzyme catalytic rate. J Lab Clin Med. 1982 Apr;99(4):495-511. [6174658 ]
- Tax WJ, Veerkamp JH: A simple and sensitive method for estimating the concentration and synthesis of 5-phosphoribosyl 1-pyrophosphate in red blood cells. Clin Chim Acta. 1977 Jul 15;78(2):209-16. [195752 ]
- MacDermot KD, Allsop J, Watts RW: The rate of purine synthesis de nova in blood mononuclear cells in vitro from patients with familial hyperuricaemic nephropathy. Clin Sci (Lond). 1984 Aug;67(2):249-58. [6744792 ]
- Emmerson BT, Gordon RB, Thompson L: Adenine phosphoribosyltransferase deficiency: its inheritance and occurrence in a female with gout and renal disease. Aust N Z J Med. 1975 Oct;5(5):440-6. [1061547 ]
- Zerez CR, Lachant NA, Tanaka KR: Decreased erythrocyte phosphoribosylpyrophosphate synthetase activity and impaired formation in thalassemia minor: a mechanism for decreased adenine nucleotide content. J Lab Clin Med. 1989 Jul;114(1):43-50. [2544652 ]
- Rylance HJ, Wallace RC, Nuki G: A method for the determination of 5-phosphoribosyl 1-pyrophosphate concentrations in erythrocytes using high-performance liquid chromatography. Anal Biochem. 1987 Feb 1;160(2):337-41. [2437821 ]
- Kane MA, Roth E, Raptis G, Schreiber C, Waxman S: Effect of intracellular folate concentration on the modulation of 5-fluorouracil cytotoxicity by the elevation of phosphoribosylpyrophosphate in cultured human KB cells. Cancer Res. 1987 Dec 15;47(24 Pt 1):6444-50. [2445472 ]
- Lachant NA, Zerez CR, Tanaka KR: Pyrimidine nucleotides impair phosphoribosylpyrophosphate (PRPP) synthetase subunit aggregation by sequestering magnesium. A mechanism for the decreased PRPP synthetase activity in hereditary erythrocyte pyrimidine 5'-nucleotidase deficiency. Biochim Biophys Acta. 1989 Jan 19;994(1):81-8. [2535789 ]
|
|---|
| Synthesis Reference: |
Gross, Akiva; Abril, Obsidiana; Lewis, Jerome M.; Geresh, Shimona; Whitesides, George M. Practical synthesis of 5-phospho-D-ribosyl a-1-pyrophosphate (PRPP): enzymatic routes from ribose 5-phosphate or ribose. Journal of the American Chemical Society (1983), 105(25), 7428-35. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|