|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110534 |
|---|
|
Identification |
|---|
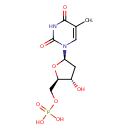
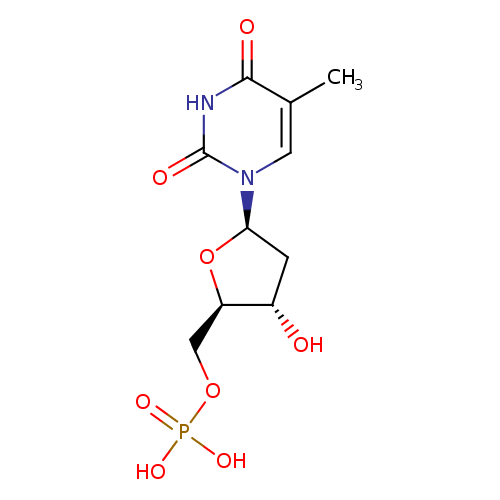
| Name: |
dTMP |
|---|
| Description: | The organophosphate oxoanion that is the dianion of dTMP arising from deprotonation of the phosphate OH groups; major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
T
-
thymidylic acid
-
thymidine monophosphate
-
deoxythymidylate
-
TMP
-
thymidine 5'-monophosphate
-
thymidine-phosphate
-
thymidine 5'-phosphate
-
Deoxythymidine 5'-phosphate
-
5'-Thymidylic acid
-
Deoxythymidylic acid
-
Thymidylate
|
|---|
|
Chemical Formula: |
C10H13N2O8P
|
|---|
| Average Molecular Weight: |
320.2 |
|---|
| Monoisotopic Molecular
Weight: |
322.0566019787 |
|---|
| InChI Key: |
GYOZYWVXFNDGLU-XLPZGREQSA-L |
|---|
| InChI: |
InChI=1S/C10H15N2O8P/c1-5-3-12(10(15)11-9(5)14)8-2-6(13)7(20-8)4-19-21(16,17)18/h3,6-8,13H,2,4H2,1H3,(H,11,14,15)(H2,16,17,18)/p-2/t6-,7+,8+/m0/s1 |
|---|
| CAS
number: |
365-07-1 |
|---|
| IUPAC Name: | 5'-O-phosphonatothymidine |
|---|
|
Traditional IUPAC Name: |
thymidylate |
|---|
| SMILES: | CC1(=CN(C(=O)NC(=O)1)C2(CC(O)C(COP(=O)([O-])[O-])O2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as pyrimidine 2'-deoxyribonucleoside monophosphates. These are pyrimidine nucleotides with a monophosphate group linked to the ribose moiety lacking a hydroxyl group at position 2. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
|
Class |
Pyrimidine nucleotides |
|---|
| Sub Class | Pyrimidine deoxyribonucleotides |
|---|
|
Direct Parent |
Pyrimidine 2'-deoxyribonucleoside monophosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrimidine 2'-deoxyribonucleoside monophosphate
- Pyrimidone
- Monoalkyl phosphate
- Hydropyrimidine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Alkyl phosphate
- Heteroaromatic compound
- Vinylogous amide
- Tetrahydrofuran
- Lactam
- Secondary alcohol
- Urea
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Alcohol
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-001i-9110000000-a45c0d5a58cdb0e5fee4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-001i-9000000000-624fe22ca203d0e6430e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-001i-9000000000-0c23943cc868acaaeda4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-004i-9703000000-452b674ca61adb40209b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Gribaudo G, Riera L, Caposio P, Maley F, Landolfo S: Human cytomegalovirus requires cellular deoxycytidylate deaminase for replication in quiescent cells. J Gen Virol. 2003 Jun;84(Pt 6):1437-41. [12771412 ]
- Seno T, Ayusawa D, Shimizu K, Koyama H, Takeishi K, Hori T: Thymineless death and genetic events in mammalian cells. Basic Life Sci. 1985;31:241-63. [3888175 ]
- Terai C, Carson DA: Pyrimidine nucleotide and nucleic acid synthesis in human monocytes and macrophages. Exp Cell Res. 1991 Apr;193(2):375-81. [1706277 ]
- Gribaudo G, Riera L, Lembo D, De Andrea M, Gariglio M, Rudge TL, Johnson LF, Landolfo S: Murine cytomegalovirus stimulates cellular thymidylate synthase gene expression in quiescent cells and requires the enzyme for replication. J Virol. 2000 Jun;74(11):4979-87. [10799571 ]
|
|---|
| Synthesis Reference: |
Scarano, E. Enzymic formation of thymidylic acid from 5-methyl-5'-deoxycytidylic acid. Bollettino - Societa Italiana di Biologia Sperimentale (1958), 34 945. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|