|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110526 |
|---|
|
Identification |
|---|
| Name: |
CMP |
|---|
| Description: | A nucleoside 5'-monophosphate(2−) that results from the removal of two protons from the phosphate group of CMP; major species at pH 7.3. |
|---|
|
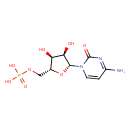
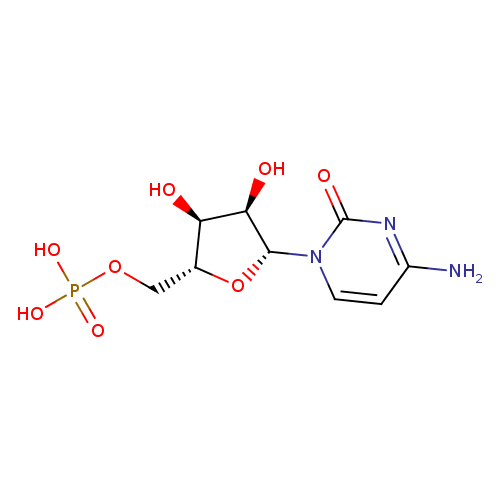
Structure |
|
|---|
| Synonyms: | -
5'-cytidylic acid
-
cytidine 5'-monophosphate
-
cytidylate
-
cytidylic acid
-
C
-
cytidine-P
-
cytidine-monophosphate
-
cytidine-phosphate
-
cytidine 5'-phosphate
|
|---|
|
Chemical Formula: |
C9H12N3O8P
|
|---|
| Average Molecular Weight: |
321.18 |
|---|
| Monoisotopic Molecular
Weight: |
323.0518509518 |
|---|
| InChI Key: |
IERHLVCPSMICTF-XVFCMESISA-L |
|---|
| InChI: |
InChI=1S/C9H14N3O8P/c10-5-1-2-12(9(15)11-5)8-7(14)6(13)4(20-8)3-19-21(16,17)18/h1-2,4,6-8,13-14H,3H2,(H2,10,11,15)(H2,16,17,18)/p-2/t4-,6-,7-,8-/m1/s1 |
|---|
| CAS
number: |
63-37-6 |
|---|
| IUPAC Name: | 5'-O-phosphonatocytidine |
|---|
|
Traditional IUPAC Name: |
cytidine monophosphate |
|---|
| SMILES: | C(C2(C(C(C(N1(C(N=C(C=C1)N)=O))O2)O)O))OP([O-])([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pyrimidine ribonucleoside monophosphates. These are pyrimidine ribobucleotides with monophosphate group linked to the ribose moiety. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Pyrimidine nucleotides |
|---|
|
Direct Parent |
Pyrimidine ribonucleoside monophosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrimidine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- Pentose monosaccharide
- Monosaccharide phosphate
- Aminopyrimidine
- Pyrimidone
- Monoalkyl phosphate
- Hydropyrimidine
- Phosphoric acid ester
- Primary aromatic amine
- Monosaccharide
- Imidolactam
- Alkyl phosphate
- Organic phosphoric acid derivative
- Pyrimidine
- Heteroaromatic compound
- Oxolane
- Secondary alcohol
- 1,2-diol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Amine
- Alcohol
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
233 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 233 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Pantothenate and CoA Biosynthesis pae00770
- Phosphatidylinositol Phosphate Metabolism pae00562
- Pyrimidine Metabolism pae00240
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-03di-0902000000-617172aee10f02d2ad2b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-03di-0900000000-ae1e8fc6db6222910637 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-03di-2900000000-c9ec8c41f82341b9225e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0229-0409000000-f28cc32ecb27c7712081 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) 30V, Positive | splash10-03di-0902000000-9a79f63e713af4d035ab | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-03di-1900000000-a5ef2297cb197b2d13e2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-00ba-9003000000-fed24d718a29a060134b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-00ba-9003000000-331ea098a652ec08d560 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. [19212411 ]
- Hirono A, Fujii H, Miyajima H, Kawakatsu T, Hiyoshi Y, Miwa S: Three families with hereditary hemolytic anemia and pyrimidine 5'-nucleotidase deficiency: electrophoretic and kinetic studies. Clin Chim Acta. 1983 May 30;130(2):189-97. [6307548 ]
- Okahira S, Nishikawa F, Nishikawa S, Akazawa T, Seya T, Matsumoto M: Interferon-beta induction through toll-like receptor 3 depends on double-stranded RNA structure. DNA Cell Biol. 2005 Oct;24(10):614-23. [16225392 ]
- Daunter B, Newlands J: Seminal plasma biochemistry II: seminal plasma and spermatozoal cytidine monophosphate-sialic acid synthetase and sialyltransferase activities. Andrologia. 1981 May-Jun;13(3):215-24. [6267956 ]
- Schmukler M, Jewett PB, Levy CC: The effects of polyamines on a residue-specific human plasma ribonuclease. J Biol Chem. 1975 Mar 25;250(6):2206-12. [234961 ]
- Paglia DE, Valentine WN, Keitt AS, Brockway RA, Nakatani M: Pyrimidine nucleotidase deficiency with active dephosphorylation of dTMP: evidence for existence of thymidine nucleotidase in human erythrocytes. Blood. 1983 Nov;62(5):1147-9. [6313098 ]
- Yates AJ, Warner JK: Behavior of sugar derivatives in procedures for ganglioside isolation. Lipids. 1984 Jul;19(7):562-9. [6748871 ]
- Li YP, Curley G, Lopez M, Chavez M, Glew R, Aragon A, Kumar H, Baca OG: Protein-tyrosine phosphatase activity of Coxiella burnetii that inhibits human neutrophils. Acta Virol. 1996 Nov-Dec;40(5-6):263-72. [9171454 ]
- Hirono A, Fujii H, Natori H, Kurokawa I, Miwa S: Chromatographic analysis of human erythrocyte pyrimidine 5'-nucleotidase from five patients with pyrimidine 5'-nucleotidase deficiency. Br J Haematol. 1987 Jan;65(1):35-41. [3028466 ]
- Gella A, Ponce J, Cusso R, Durany N: Effect of the nucleotides CMP and UMP on exhaustion in exercise rats. J Physiol Biochem. 2008 Mar;64(1):9-17. [18663991 ]
|
|---|
| Synthesis Reference: |
Zhou, Jingkang. Method for preparing cytidine 5'-monophosphate. Faming Zhuanli Shenqing Gongkai Shuomingshu (2005), 12 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|