|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110524 |
|---|
|
Identification |
|---|
| Name: |
UTP |
|---|
| Description: | A nucleoside triphosphate(4−) obtained by global deprotonation of the triphosphate OH groups of UTP; major species present at pH 7.3. |
|---|
|
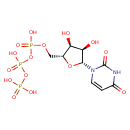
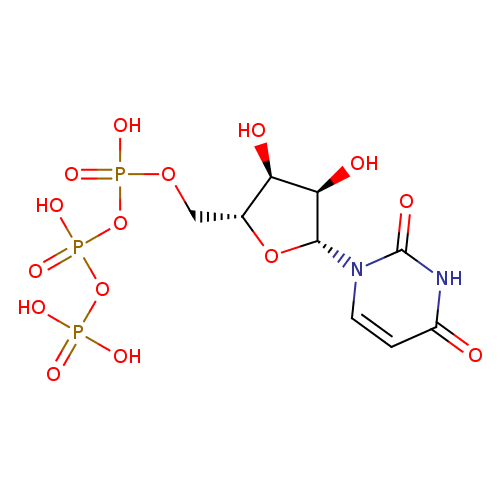
Structure |
|
|---|
| Synonyms: | -
uridine-triphosphate
-
uridine-5'-triphosphate
|
|---|
|
Chemical Formula: |
C9H11N2O15P3
|
|---|
| Average Molecular Weight: |
480.11 |
|---|
| Monoisotopic Molecular
Weight: |
483.9685273534 |
|---|
| InChI Key: |
PGAVKCOVUIYSFO-XVFCMESISA-J |
|---|
| InChI: |
InChI=1S/C9H15N2O15P3/c12-5-1-2-11(9(15)10-5)8-7(14)6(13)4(24-8)3-23-28(19,20)26-29(21,22)25-27(16,17)18/h1-2,4,6-8,13-14H,3H2,(H,19,20)(H,21,22)(H,10,12,15)(H2,16,17,18)/p-4/t4-,6-,7-,8-/m1/s1 |
|---|
| CAS
number: |
63-39-8 |
|---|
| IUPAC Name: | uridine 5'-triphosphate(4−) |
|---|
|
Traditional IUPAC Name: |
uridine 5'-triphosphoric acid |
|---|
| SMILES: | C(OP(=O)([O-])OP(=O)([O-])OP(=O)([O-])[O-])C1(OC(C(O)C(O)1)N2(C=CC(=O)NC(=O)2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pyrimidine ribonucleoside triphosphates. These are pyrimidine ribobucleotides with triphosphate group linked to the ribose moiety. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Pyrimidine nucleotides |
|---|
|
Direct Parent |
Pyrimidine ribonucleoside triphosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrimidine ribonucleoside triphosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Hydroxypyrimidine
- Pyrimidone
- Monoalkyl phosphate
- Hydropyrimidine
- Monosaccharide
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Alkyl phosphate
- Heteroaromatic compound
- Oxolane
- Secondary alcohol
- 1,2-diol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kunzelmann K, Mall M: Pharmacotherapy of the ion transport defect in cystic fibrosis: role of purinergic receptor agonists and other potential therapeutics. Am J Respir Med. 2003;2(4):299-309. [14719996 ]
- Erlinge D, Harnek J, van Heusden C, Olivecrona G, Jern S, Lazarowski E: Uridine triphosphate (UTP) is released during cardiac ischemia. Int J Cardiol. 2005 Apr 28;100(3):427-33. [15837087 ]
- Oosterhuis GJ, Mulder AB, Kalsbeek-Batenburg E, Lambalk CB, Schoemaker J, Vermes I: Measuring apoptosis in human spermatozoa: a biological assay for semen quality? Fertil Steril. 2000 Aug;74(2):245-50. [10927039 ]
- Holstege A, Manglitz D, Gerok W: Depletion of blood plasma cytidine due to increased hepatocellular salvage in D-galactosamine-treated rats. Eur J Biochem. 1984 Jun 1;141(2):339-44. [6734601 ]
|
|---|
| Synthesis Reference: |
Kenner, G. W.; Todd, A. R.; Webb, R. F.; Weymouth, F. J. Nucleotides. XXVIII. Synthesis of uridine 5'-triphosphate. Journal of the Chemical Society (1954), 46-52 2288-93. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|