|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110513 |
|---|
|
Identification |
|---|
| Name: |
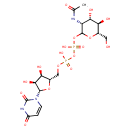
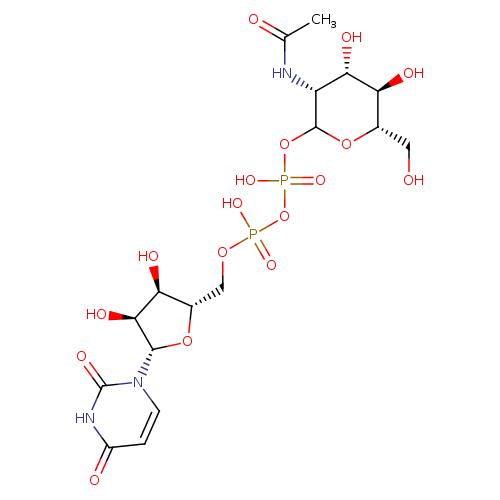
UDP-N-acetyl-α-D-mannosamine |
|---|
| Description: | A UDP-N-acetyl-D-mannosamine(2−) in which the anomeric centre of the pyranose fragment has α-configuration. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
uridine diphosphate N-acetylmannosamine
-
uridine 5'-(trihydrogen diphosphate)
- P'-(2-(acetylamino)-2-deoxy-α-D-mannopyranosyl) ester
-
UDP-acetylmannosamine
-
UDP-ManNAc
|
|---|
|
Chemical Formula: |
C17H25N3O17P2
|
|---|
| Average Molecular Weight: |
605.34 |
|---|
| Monoisotopic Molecular
Weight: |
607.081569478 |
|---|
| InChI Key: |
LFTYTUAZOPRMMI-ZYQOOJPVSA-L |
|---|
| InChI: |
InChI=1S/C17H27N3O17P2/c1-6(22)18-10-13(26)11(24)7(4-21)35-16(10)36-39(31,32)37-38(29,30)33-5-8-12(25)14(27)15(34-8)20-3-2-9(23)19-17(20)28/h2-3,7-8,10-16,21,24-27H,4-5H2,1H3,(H,18,22)(H,29,30)(H,31,32)(H,19,23,28)/p-2/t7-,8-,10+,11-,12-,13-,14-,15-,16-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | thymidine 5'-[3-(2-acetamido-2-deoxy-α-D-mannopyranosyl) diphosphate] |
|---|
|
Traditional IUPAC Name: |
{[(2S,3R,4S,5S)-5-(2,4-dioxo-3H-pyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy[(3R,4S,5R,6S)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphosphinic acid |
|---|
| SMILES: | CC(=O)NC3(C(O)C(O)C(CO)OC(OP(=O)([O-])OP(=O)([O-])OCC1(OC(C(O)C(O)1)N2(C=CC(=O)NC(=O)2)))3) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pyrimidine nucleotide sugars. These are pyrimidine nucleotides bound to a saccharide derivative through the terminal phosphate group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Pyrimidine nucleotides |
|---|
|
Direct Parent |
Pyrimidine nucleotide sugars |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrimidine nucleotide sugar
- Pyrimidine ribonucleoside diphosphate
- Pentose phosphate
- Pentose-5-phosphate
- N-acyl-alpha-hexosamine
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Organic pyrophosphate
- Pyrimidone
- Monoalkyl phosphate
- Hydropyrimidine
- Monosaccharide
- Organic phosphoric acid derivative
- Oxane
- Phosphoric acid ester
- Pyrimidine
- Alkyl phosphate
- Vinylogous amide
- Acetamide
- Tetrahydrofuran
- Heteroaromatic compound
- Urea
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Lactam
- Azacycle
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Organopnictogen compound
- Organooxygen compound
- Alcohol
- Primary alcohol
- Organic nitrogen compound
- Organic oxygen compound
- Organic oxide
- Carbonyl group
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- UDP-N-acetyl-α-D-mannosaminouronate biosynthesisPWY-7335
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Samuel J, Tanner ME (2004)Active site mutants of the "non-hydrolyzing" UDP-N-acetylglucosamine 2-epimerase from Escherichia coli. Biochimica et biophysica acta 1700, Pubmed: 15210128
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|