|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110489 |
|---|
|
Identification |
|---|
| Name: |
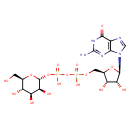
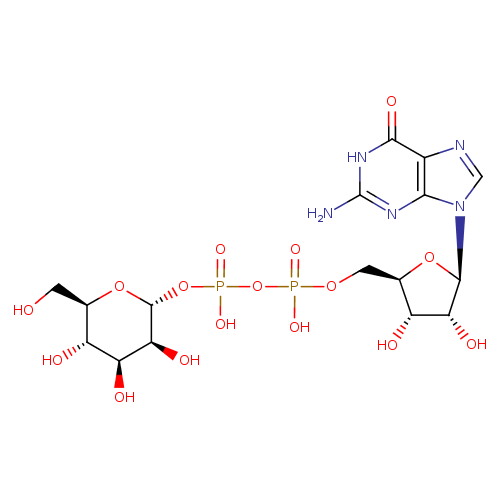
GDP-α-D-mannose |
|---|
| Description: | Conjugate base of GDP-α-D-mannose arising from deprotonation of both free OH groups of the diphosphate. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
guanosine pyrophosphate mannose
-
guanosine diphosphomannose
-
guanosine diphosphate mannose
-
GDP-mannose
|
|---|
|
Chemical Formula: |
C16H23N5O16P2
|
|---|
| Average Molecular Weight: |
603.33 |
|---|
| Monoisotopic Molecular
Weight: |
605.0771528021 |
|---|
| InChI Key: |
MVMSCBBUIHUTGJ-GDJBGNAASA-L |
|---|
| InChI: |
InChI=1S/C16H25N5O16P2/c17-16-19-12-6(13(28)20-16)18-3-21(12)14-10(26)8(24)5(34-14)2-33-38(29,30)37-39(31,32)36-15-11(27)9(25)7(23)4(1-22)35-15/h3-5,7-11,14-15,22-27H,1-2H2,(H,29,30)(H,31,32)(H3,17,19,20,28)/p-2/t4-,5-,7-,8-,9+,10-,11+,14-,15-/m1/s1 |
|---|
| CAS
number: |
3123-67-9 |
|---|
| IUPAC Name: | guanosine 5'-[3-(α-D-mannopyranosyl) diphosphate] |
|---|
|
Traditional IUPAC Name: |
guanosine diphosphomannose |
|---|
| SMILES: | C(OP(=O)([O-])OP(=O)([O-])OC1(OC(C(O)C(O)C(O)1)CO))C2(C(O)C(O)C(O2)N4(C=NC3(C(=O)NC(N)=NC=34))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as purine nucleotide sugars. These are purine nucleotides bound to a saccharide derivative through the terminal phosphate group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
Purine nucleotide sugars |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine nucleotide sugar
- Purine ribonucleoside diphosphate
- Purine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Organic pyrophosphate
- Imidazopyrimidine
- Purine
- Hydroxypyrimidine
- Monoalkyl phosphate
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Oxane
- Phosphoric acid ester
- Alkyl phosphate
- Pyrimidine
- Heteroaromatic compound
- Oxolane
- Imidazole
- Azole
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Azacycle
- Polyol
- Organopnictogen compound
- Alcohol
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Organonitrogen compound
- Organooxygen compound
- Organic nitrogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Fructose and Mannose Degradation pae00051
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Becker DJ, Lowe JB: Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003 Jul;13(7):41R-53R. Epub 2003 Mar 19. [12651883 ]
|
|---|
| Synthesis Reference: |
Huang, Gang-Liang; Liu, Xiang; Zhang, Hou-Cheng; Wang, Peng-George. A facile two-step chemo-enzymatic synthesis of GDP-mannose. Letters in Organic Chemistry (2006), 3(9), 668-669. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|