|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110476 |
|---|
|
Identification |
|---|
| Name: |
cyclic-AMP |
|---|
| Description: | An organophosphate oxoanion that is the conjugate base of 3',5'-cyclic AMP arising from deprotonation of the free phosphate OH group; major species at pH 7.3. |
|---|
|
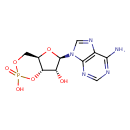
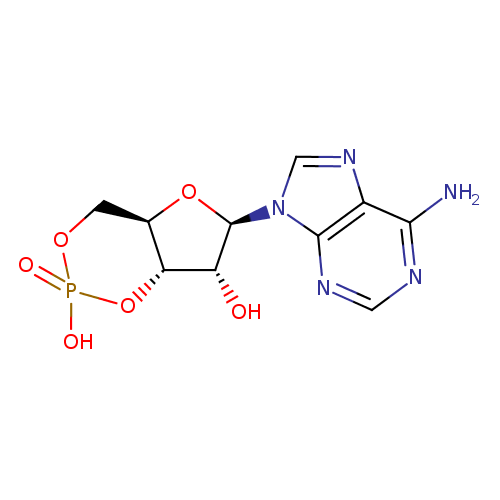
Structure |
|
|---|
| Synonyms: | -
cyclic 3',5'-AMP
-
3',5'-cyclic AMP
-
adenosine cyclic-3',5'-monophosphate
-
adenosine cyclic-monophosphate
-
adenosine-cyclic-phosphoric-acid
-
cAMP
-
adenosine-cyclic-phosphate
-
adenosine-3',5'-monophosphate
-
adenosine 3',5'-cyclic phosphate
-
adenosine-3',5'-cyclic monophosphate
-
cyclic-3',5'-adenosine monophosphate
|
|---|
|
Chemical Formula: |
C10H11N5O6P
|
|---|
| Average Molecular Weight: |
328.2 |
|---|
| Monoisotopic Molecular
Weight: |
329.0525196538 |
|---|
| InChI Key: |
IVOMOUWHDPKRLL-KQYNXXCUSA-M |
|---|
| InChI: |
InChI=1S/C10H12N5O6P/c11-8-5-9(13-2-12-8)15(3-14-5)10-6(16)7-4(20-10)1-19-22(17,18)21-7/h2-4,6-7,10,16H,1H2,(H,17,18)(H2,11,12,13)/p-1/t4-,6-,7-,10-/m1/s1 |
|---|
| CAS
number: |
60-92-4 |
|---|
| IUPAC Name: | adenosine 3',5'-phosphate |
|---|
|
Traditional IUPAC Name: |
cyclic adenosine monophosphate |
|---|
| SMILES: | C1(OP(=O)([O-])OC2(C(O)C(OC12)N4(C=NC3(C(N)=NC=NC=34)))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as 3',5'-cyclic purine nucleotides. These are purine nucleotides in which the oxygen atoms linked to the C3 and C5 carbon atoms of the ribose moiety are both bonded the same phosphorus atom of the phosphate group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
|
Class |
Purine nucleotides |
|---|
| Sub Class | Cyclic purine nucleotides |
|---|
|
Direct Parent |
3',5'-cyclic purine nucleotides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 3',5'-cyclic purine ribonucleotide
- Pentose phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Primary aromatic amine
- Pyrimidine
- Imidolactam
- Oxolane
- Azole
- Imidazole
- Heteroaromatic compound
- Secondary alcohol
- Azacycle
- Organoheterocyclic compound
- Oxacycle
- Primary amine
- Organopnictogen compound
- Organic nitrogen compound
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organonitrogen compound
- Amine
- Organooxygen compound
- Organic anion
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
- a nucleoside 3',5'-cyclic phosphate, a purine-related compound (CAMP)
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
219 - 220 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 219 - 220 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 4 mg/mL | Not Available | | LogP | -2.96 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-9761000000-ed8f1fea0ef2e7b43ff8 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-03du-2971000000-c0200940e0e88308fbde | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-004i-0009000000-02ee7f342f5c1bada389 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-004i-0009000000-a00a2165c3d37c4e1ef2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-004i-0109000000-7f48145f84828c2bbd5d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-001i-0119000000-d94a34a13521ae3d4692 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0udi-0900000000-f10e1d70e30865424af6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-03dr-0629000000-bf4ec1094b7991c3633c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-03dr-0629000000-9736faaf70ec79d5bd03 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-001i-0109000000-6bf3784f4b710ce04a3e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-03dr-0629000000-3f5ea9ba1508e359ad80 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-03dr-0519000000-059e8569ee179529bf21 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-001i-0009000000-88dad10a362b1088849e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-004i-0509004000-bb02289fcd39ac1a203c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0a4i-1900000000-da49323734f24bf724d6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-001i-0900000000-a4e60131f41413b6fecf | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-004i-0009000000-98ee4e70689c06adc603 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-056r-0409005001-97fd793d1d14a84c3530 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0a4i-0900000000-1f2045ac2ff736aa80a2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-001i-0900000000-8bbb24702fa6ccd76268 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-004i-0009000000-6d23df7535894180e6df | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-001i-0009000000-c527aad341e122fc9bea | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) 30V, Positive | splash10-001i-0209000000-1bb2d660cc00c85a808f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-003r-2904000000-865c1b989665f73ac3f4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Butt E, Beltman J, Becker DE, Jensen GS, Rybalkin SD, Jastorff B, Beavo JA (1995)Characterization of cyclic nucleotide phosphodiesterases with cyclic AMP analogs: topology of the catalytic sites and comparison with other cyclic AMP-binding proteins. Molecular pharmacology 47, Pubmed: 7870042
- Beltman J, Becker DE, Butt E, Jensen GS, Rybalkin SD, Jastorff B, Beavo JA (1995)Characterization of cyclic nucleotide phosphodiesterases with cyclic GMP analogs: topology of the catalytic domains. Molecular pharmacology 47, Pubmed: 7870041
|
|---|
| Synthesis Reference: |
Genieser, H. G.; Butt, E.; Bottin, U.; Dostmann, W.; Jastorff, B. Synthesis of the 3',5'-cyclic phosphates from unprotected nucleosides. Synthesis (1989), (1), 53-4. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|