|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110474 |
|---|
|
Identification |
|---|
| Name: |
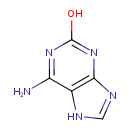
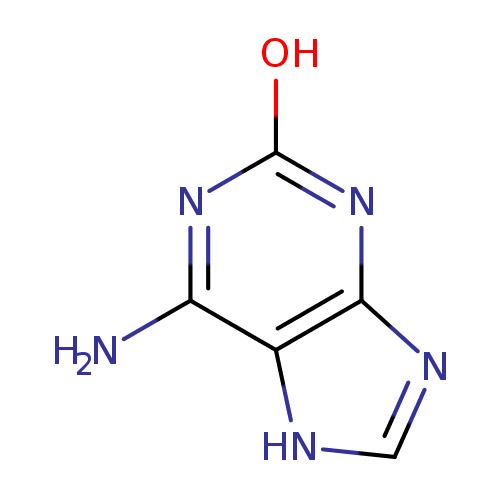
isoguanine |
|---|
| Description: | An oxopurine that is 3,7-dihydro-purin-2-one in which the hydrogen at position 6 is substituted by an amino group. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
2-oxoadenine
-
2-hydroxyadenine
|
|---|
|
Chemical Formula: |
C5H5N5O
|
|---|
| Average Molecular Weight: |
151.0494098086 |
|---|
| Monoisotopic Molecular
Weight: |
151.0494098086 |
|---|
| InChI Key: |
DRAVOWXCEBXPTN-UHFFFAOYSA-N |
|---|
| InChI: |
InChI=1S/C5H5N5O/c6-3-2-4(8-1-7-2)10-5(11)9-3/h1H,(H4,6,7,8,9,10,11) |
|---|
| CAS
number: |
3373-53-3 |
|---|
| IUPAC Name: | 6-amino-3,7-dihydro-2H-purin-2-one |
|---|
|
Traditional IUPAC Name: |
2-hydroxy-6-aminopurine |
|---|
| SMILES: | C2(=NC1(=C(NC(N=C(N)1)=O)N2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as 6-aminopurines. These are purines that carry an amino group at position 6. Purine is a bicyclic aromatic compound made up of a pyrimidine ring fused to an imidazole ring. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Imidazopyrimidines |
|---|
|
Direct Parent |
6-aminopurines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 6-aminopurine
- Aminopyrimidine
- Hydroxypyrimidine
- Primary aromatic amine
- Pyrimidine
- Imidolactam
- Azole
- Imidazole
- Heteroaromatic compound
- Azacycle
- Organopnictogen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Amine
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
> 360 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | > 360 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.0625 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kamiya H, Kasai H (1995)2-Hydroxyadenine (isoguanine) as oxidative DNA damage: its formation and mutation inducibility. Nucleic acids symposium series233-234 [PubMed:8841637][show Abstract]In our experiments we found that hydroxylation occurs at the C-2 position of adenine by oxygen radical treatment (Fe(2+)-EDTA) of dA, dATP, and single- and double-stranded DNA. This oxidatively damaged base, 2-hydroxyadenine (also known as isoguanine), was produced more efficiently in monomers than in polynucleotides. 2-Hydroxydeoxyadenosine triphosphate was incorporated opposite T and C in a DNA template by DNA polymerase alpha and only opposite T by the Klenow fragment. The Klenow fragment, DNA polymerases alpha and beta incorporated dTMP and other nucleotides opposite 2-OH-Ade in DNA templates in vitro in a sequence-dependent manner. These results suggest that the formation of 2-OH-Ade in DNA will induce mutations in cells.
|
|---|
| Rogstad KN, Jang YH, Sowers LC, Goddard WA (2003)First principles calculations of the pKa values and tautomers of isoguanine and xanthine. Chemical research in toxicology 16, Pubmed: 14615972 Blas JR, Luque FJ, Orozco M (2004)Unique tautomeric properties of isoguanine. Journal of the American Chemical Society 126, Pubmed: 14709079 Yang XL, Sugiyama H, Ikeda S, Saito I, Wang AH (1998)Structural studies of a stable parallel-stranded DNA duplex incorporating isoguanine:cytosine and isocytosine:guanine basepairs by nuclear magnetic resonance spectroscopy. Biophysical journal 75, Pubmed: 9726918 Pettit GR, Ode RH, Coomes RM, Ode SL (1976)Antineoplastic agents. 42. The butterfly, Prioneris thestylis. Lloydia 39, Pubmed: 1018622 |
| Synthesis Reference: |
Hayashi, Taketo; Tamato, Toyomochi. Preparation of isoguanine. Jpn. Kokai Tokkyo Koho (1994), 3 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|