|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110473 |
|---|
|
Identification |
|---|
| Name: |
GDP |
|---|
| Description: | Trianion of guanosine 5'-diphosphate arising from deprotonation of the three diphosphate OH groups; major species at pH 7.3. |
|---|
|
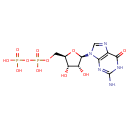
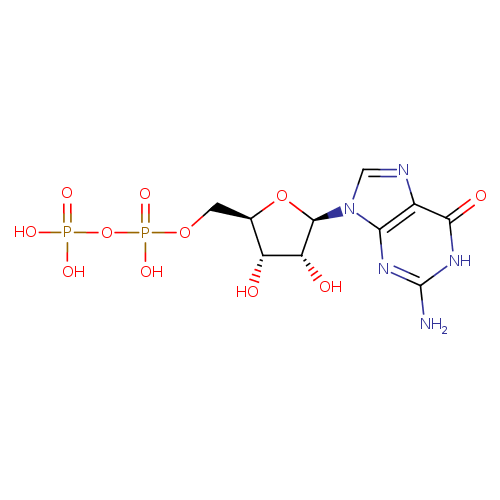
Structure |
|
|---|
| Synonyms: | -
ppG
-
guanosine-5'-diphosphate
-
guanosine-diphosphate
|
|---|
|
Chemical Formula: |
C10H12N5O11P2
|
|---|
| Average Molecular Weight: |
440.18 |
|---|
| Monoisotopic Molecular
Weight: |
443.0243293706 |
|---|
| InChI Key: |
QGWNDRXFNXRZMB-UUOKFMHZSA-K |
|---|
| InChI: |
InChI=1S/C10H15N5O11P2/c11-10-13-7-4(8(18)14-10)12-2-15(7)9-6(17)5(16)3(25-9)1-24-28(22,23)26-27(19,20)21/h2-3,5-6,9,16-17H,1H2,(H,22,23)(H2,19,20,21)(H3,11,13,14,18)/p-3/t3-,5-,6-,9-/m1/s1 |
|---|
| CAS
number: |
146-91-8 |
|---|
| IUPAC Name: | 5'-O-[(phosphonatooxy)phosphinato]guanosine |
|---|
|
Traditional IUPAC Name: |
GDP |
|---|
| SMILES: | C(OP(=O)([O-])OP(=O)([O-])[O-])C1(OC(C(O)C(O)1)N3(C=NC2(C(=O)NC(N)=NC=23))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as purine ribonucleoside diphosphates. These are purine ribobucleotides with diphosphate group linked to the ribose moiety. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
Purine ribonucleoside diphosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine ribonucleoside diphosphate
- Purine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-oxopurine
- Hypoxanthine
- Monosaccharide phosphate
- Organic pyrophosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Pyrimidone
- Monoalkyl phosphate
- Aminopyrimidine
- Pyrimidine
- Alkyl phosphate
- N-substituted imidazole
- Primary aromatic amine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Monosaccharide
- Vinylogous amide
- Heteroaromatic compound
- Imidazole
- Oxolane
- Azole
- Secondary alcohol
- 1,2-diol
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Alcohol
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Primary amine
- Organic nitrogen compound
- Amine
- Organooxygen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0udl-0900600000-5c7173a5771dfefadc5e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0udi-0900000000-d38af29994fa5108c331 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0udi-0900000000-3072d857dcc8a1b2cbaa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Kim HY, Thomas D, Hanley MR (1996)Stimulation of Ca(2+)-dependent membrane currents in Xenopus oocytes by microinjection of pyrimidine nucleotide-glucose conjugates. Molecular pharmacology 49, Pubmed: 8632770
- Wennefors CK, Dobrikov MI, Xu Z, Li P, Shaw BR (2008)Stereospecificity, substrate, and inhibitory properties of nucleoside diphosphate analogs for creatine and pyruvate kinases. Bioorganic chemistry 36, Pubmed: 18433830
|
|---|
| Synthesis Reference: |
Edlin, Gordon; Donini, P. Synthesis of guanosine 5'-diphosphate, 2'-(or 3'-) diphosphate, and related nucleotides in a variety of physiological conditions. Journal of Biological Chemistry (1971), 246(13), 4371-3. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|