|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110471 |
|---|
|
Identification |
|---|
| Name: |
GMP |
|---|
| Description: | A nucleoside 5'-monophosphate(2−) that results from the removal of two protons from the phosphate group of GMP. |
|---|
|
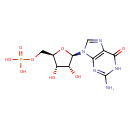
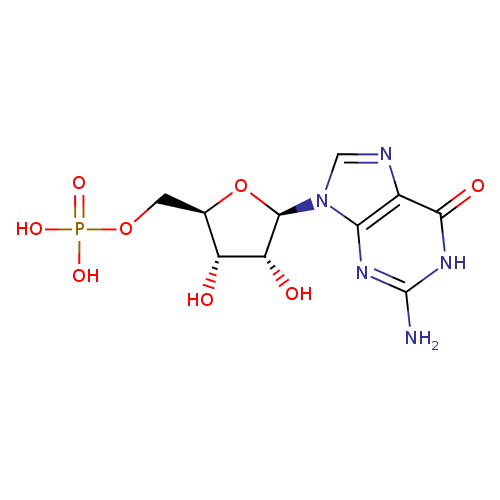
Structure |
|
|---|
| Synonyms: | -
guanylate
-
G
-
guanylic acid
-
guanosine phosphate
-
guanosine 5'-phosphate
-
guanosine monophosphate
-
guanosine-5'-monophosphate
|
|---|
|
Chemical Formula: |
C10H12N5O8P
|
|---|
| Average Molecular Weight: |
361.21 |
|---|
| Monoisotopic Molecular
Weight: |
363.0579989622 |
|---|
| InChI Key: |
RQFCJASXJCIDSX-UUOKFMHZSA-L |
|---|
| InChI: |
InChI=1S/C10H14N5O8P/c11-10-13-7-4(8(18)14-10)12-2-15(7)9-6(17)5(16)3(23-9)1-22-24(19,20)21/h2-3,5-6,9,16-17H,1H2,(H2,19,20,21)(H3,11,13,14,18)/p-2/t3-,5-,6-,9-/m1/s1 |
|---|
| CAS
number: |
85-32-5 |
|---|
| IUPAC Name: | 5'-O-phosphonatoguanosine |
|---|
|
Traditional IUPAC Name: |
guanylate |
|---|
| SMILES: | C(OP([O-])(=O)[O-])C1(OC(C(O)C(O)1)N3(C=NC2(C(=O)NC(N)=NC=23))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as purine ribonucleoside monophosphates. These are nucleotides consisting of a purine base linked to a ribose to which one monophosphate group is attached. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
|
Class |
Purine nucleotides |
|---|
| Sub Class | Purine ribonucleotides |
|---|
|
Direct Parent |
Purine ribonucleoside monophosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine ribonucleoside monophosphate
- Pentose-5-phosphate
- Pentose phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-oxopurine
- Pentose monosaccharide
- Hypoxanthine
- Monosaccharide phosphate
- Imidazopyrimidine
- Purine
- Pyrimidone
- Aminopyrimidine
- N-substituted imidazole
- Pyrimidine
- Phosphoric acid ester
- Monosaccharide
- Alkyl phosphate
- Organic phosphoric acid derivative
- Azole
- Heteroaromatic compound
- Imidazole
- Vinylogous amide
- Oxolane
- 1,2-diol
- Secondary alcohol
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Amine
- Alcohol
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organonitrogen compound
- Organooxygen compound
- Organic oxygen compound
- Primary amine
- Organic anion
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
- a purine-related compound, a ribonucleoside monophosphate (GMP)
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 369 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (6 TMS) | splash10-014i-1942000000-27fc2e135f86071ba4d7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0udi-0901000000-fbce35f7dfa73ab5fc11 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0udi-0900000000-764722cc1b8cc2aaa480 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0udi-0900000000-c268cdce8dfd6c39fa3c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-03di-0409000000-309e438ad8b6162df850 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) 30V, Positive | splash10-0udi-0904000000-cf72d0459316801b014e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0udi-0900000000-abc7bfe7620db1c34775 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-01t9-9114000000-9a1071a2e17ea3ca268c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-01t9-9113000000-05a8989070e3b89138f1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Keough DT, Hocková D, Holý A, Naesens LM, Skinner-Adams TS, Jersey Jd, Guddat LW (2009)Inhibition of hypoxanthine-guanine phosphoribosyltransferase by acyclic nucleoside phosphonates: a new class of antimalarial therapeutics. Journal of medicinal chemistry 52, Pubmed: 19527031
|
|---|
| Synthesis Reference: |
Sato, Katsuaki; Matsui, Hiroshi; Ei, Hitoshi; Takinami, Koichi. Guanosine-5'-monophosphate. Jpn. Kokai Tokkyo Koho (1979), 3 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|