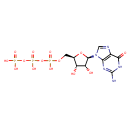|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110470 |
|---|
|
Identification |
|---|
| Name: |
GTP |
|---|
| Description: | A nucleoside triphosphate(4−) obtained by global deprotonation of the triphosphate OH groups of GTP; major species present at pH 7.3. |
|---|
|
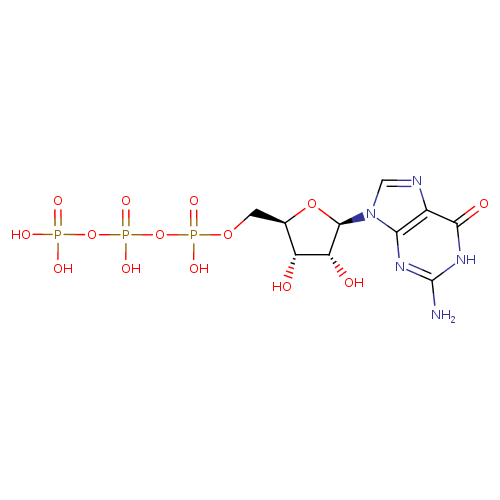
Structure |
|
|---|
| Synonyms: | -
guanylyl imidodiphosphate
-
guanosine 5'-triphosphate
-
guanosine-triphosphate
|
|---|
|
Chemical Formula: |
C10H12N5O14P3
|
|---|
| Average Molecular Weight: |
519.15 |
|---|
| Monoisotopic Molecular
Weight: |
522.990659779 |
|---|
| InChI Key: |
XKMLYUALXHKNFT-UUOKFMHZSA-J |
|---|
| InChI: |
InChI=1S/C10H16N5O14P3/c11-10-13-7-4(8(18)14-10)12-2-15(7)9-6(17)5(16)3(27-9)1-26-31(22,23)29-32(24,25)28-30(19,20)21/h2-3,5-6,9,16-17H,1H2,(H,22,23)(H,24,25)(H2,19,20,21)(H3,11,13,14,18)/p-4/t3-,5-,6-,9-/m1/s1 |
|---|
| CAS
number: |
86-01-1 |
|---|
| IUPAC Name: | guanosine 5'-triphosphate(4−) |
|---|
|
Traditional IUPAC Name: |
triphosphate, guanosine |
|---|
| SMILES: | C(OP(=O)([O-])OP(=O)([O-])OP(=O)([O-])[O-])C1(OC(C(O)C(O)1)N3(C=NC2(C(=O)NC(N)=NC=23))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as purine ribonucleoside triphosphates. These are purine ribobucleotides with a triphosphate group linked to the ribose moiety. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
Purine ribonucleoside triphosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine ribonucleoside triphosphate
- Purine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Imidazopyrimidine
- Purine
- Hydroxypyrimidine
- Monoalkyl phosphate
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Alkyl phosphate
- Azole
- Heteroaromatic compound
- Oxolane
- Imidazole
- Secondary alcohol
- 1,2-diol
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organonitrogen compound
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-006t-0000970000-ed775d45b7d4c3b969df | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0002-0000920000-eea0cfe4824f5aee7e4a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0006-0900000000-895098906ea44c18ea43 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Chantin C, Bonin B, Boulieu R, Bory C: Liquid-chromatographic study of purine metabolism abnormalities in purine nucleoside phosphorylase deficiency. Clin Chem. 1996 Feb;42(2):326-8. [8595732 ]
- Naylor EW, Ennis D, Davidson AG, Wong LT, Applegarth DA, Niederwieser A: Guanosine triphosphate cyclohydrolase I deficiency: early diagnosis by routine urine pteridine screening. Pediatrics. 1987 Mar;79(3):374-8. [3822637 ]
- Iwanaga N, Yamamasu S, Tachibana D, Nishio J, Nakai Y, Shintaku H, Ishiko O: Activity of synthetic enzymes of tetrahydrobiopterin in the human placenta. Int J Mol Med. 2004 Jan;13(1):117-20. [14654981 ]
- Lester HA, Steer ML, Levitzki A: Prostaglandin-stimulated GTP hydrolysis associated with activation of adenylate cyclase in human platelet membranes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):719-23. [6121325 ]
- Reichert LE Jr, Dattatreyamurty B: The follicle-stimulating hormone (FSH) receptor in testis: interaction with FSH, mechanism of signal transduction, and properties of the purified receptor. Biol Reprod. 1989 Jan;40(1):13-26. [2493820 ]
- Schmidt VA, Scudder L, Devoe CE, Bernards A, Cupit LD, Bahou WF: IQGAP2 functions as a GTP-dependent effector protein in thrombin-induced platelet cytoskeletal reorganization. Blood. 2003 Apr 15;101(8):3021-8. Epub 2002 Dec 19. [12515716 ]
- Chen Q, He Y, Yang K: Gene therapy for Parkinson's disease: progress and challenges. Curr Gene Ther. 2005 Feb;5(1):71-80. [15638712 ]
|
|---|
| Synthesis Reference: |
Stiller, Regine; Thiem, Joachim. Preparative enzymatic conversion of guanosine-5'-monophosphate to guanosine-5'-triphosphate. Synlett (1990), (11), 709-10. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|