|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110467 |
|---|
|
Identification |
|---|
| Name: |
IMP |
|---|
| Description: | A nucleoside 5'-monophosphate(2−) that results from the removal of two protons from the phosphate group of IMP; major species at pH 7.3. |
|---|
|
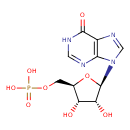
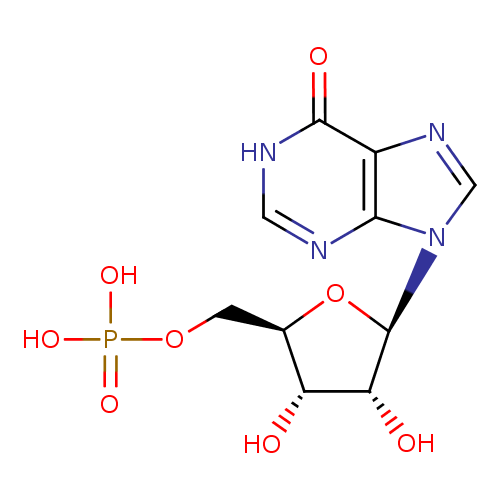
Structure |
|
|---|
| Synonyms: | -
5'-IMP
-
ribosylhypoxanthine monophosphate
-
inosinate
-
inosine monophosphate
-
inosine 5'-monophosphate
-
inosine 5'-phosphate
-
5'-inosinate
-
5'-inosinic acid
-
5'-inosine monophosphate
|
|---|
|
Chemical Formula: |
C10H11N4O8P
|
|---|
| Average Molecular Weight: |
346.19 |
|---|
| Monoisotopic Molecular
Weight: |
348.0470999249 |
|---|
| InChI Key: |
GRSZFWQUAKGDAV-KQYNXXCUSA-L |
|---|
| InChI: |
InChI=1S/C10H13N4O8P/c15-6-4(1-21-23(18,19)20)22-10(7(6)16)14-3-13-5-8(14)11-2-12-9(5)17/h2-4,6-7,10,15-16H,1H2,(H,11,12,17)(H2,18,19,20)/p-2/t4-,6-,7-,10-/m1/s1 |
|---|
| CAS
number: |
131-99-7 |
|---|
| IUPAC Name: | 5'-O-phosphonatoinosine |
|---|
|
Traditional IUPAC Name: |
inosine-5'-monophosphate |
|---|
| SMILES: | C(OP(=O)([O-])[O-])C1(OC(C(O)C(O)1)N3(C=NC2(C(=O)NC=NC=23))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as purine ribonucleoside monophosphates. These are nucleotides consisting of a purine base linked to a ribose to which one monophosphate group is attached. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
Purine ribonucleoside monophosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-oxopurine
- Hypoxanthine
- Monosaccharide phosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Pyrimidone
- Alkyl phosphate
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Heteroaromatic compound
- Oxolane
- Azole
- Imidazole
- Vinylogous amide
- Secondary alcohol
- 1,2-diol
- Lactam
- Oxacycle
- Organoheterocyclic compound
- Azacycle
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic nitrogen compound
- Organooxygen compound
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (5 TMS) | splash10-014i-1952000000-fd534f438bc14efb9a2c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-000i-0900000000-a46a4af4f25c710c773b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-000i-1900000000-e3960644419fb73668b1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0fb9-2983200000-58dfb3434545241ee7b6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-000i-1900000000-d9a723b143b346290896 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-002b-9203000000-e2ceede282569ac77de5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Nakayama Y, Kinoshita A, Tomita M: Dynamic simulation of red blood cell metabolism and its application to the analysis of a pathological condition. Theor Biol Med Model. 2005 May 9;2(1):18. [15882454 ]
- McConell GK, Shinewell J, Stephens TJ, Stathis CG, Canny BJ, Snow RJ: Creatine supplementation reduces muscle inosine monophosphate during endurance exercise in humans. Med Sci Sports Exerc. 2005 Dec;37(12):2054-61. [16331129 ]
- Castro-Gago M, Cid E, Trabazo S, Pavon P, Camina F, Rodriguez-Segade S, Einis Punal J, Rodriguez-Nunez A: Cerebrospinal fluid purine metabolites and pyrimidine bases after brief febrile convulsions. Epilepsia. 1995 May;36(5):471-4. [7614924 ]
- Green HJ, Grant SM, Phillips SM, Enns DL, Tarnopolsky MA, Sutton JR: Reduced muscle lactate during prolonged exercise following induced plasma volume expansion. Can J Physiol Pharmacol. 1997 Dec;75(12):1280-6. [9534937 ]
- Rodriguez-Nunez A, Cid E, Rodriguez-Garcia J, Camina F, Rodriguez-Segade S, Castro-Gago M: Concentrations of nucleotides, nucleosides, purine bases, oxypurines, uric acid, and neuron-specific enolase in the cerebrospinal fluid of children with sepsis. J Child Neurol. 2001 Sep;16(9):704-6. [11575617 ]
- Pouw EM, Schols AM, van der Vusse GJ, Wouters EF: Elevated inosine monophosphate levels in resting muscle of patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998 Feb;157(2):453-7. [9476857 ]
- Allison AC, Eugui EM: Purine metabolism and immunosuppressive effects of mycophenolate mofetil (MMF). Clin Transplant. 1996 Feb;10(1 Pt 2):77-84. [8680053 ]
- van Hall G, van der Vusse GJ, Soderlund K, Wagenmakers AJ: Deamination of amino acids as a source for ammonia production in human skeletal muscle during prolonged exercise. J Physiol. 1995 Nov 15;489 ( Pt 1):251-61. [8583409 ]
- McConell G, Snow RJ, Proietto J, Hargreaves M: Muscle metabolism during prolonged exercise in humans: influence of carbohydrate availability. J Appl Physiol. 1999 Sep;87(3):1083-6. [10484580 ]
- Klupp J, Pfitzmann R, Langrehr JM, Neuhaus P: Indications of mycophenolate mofetil in liver transplantation. Transplantation. 2005 Sep 27;80(1 Suppl):S142-6. [16286893 ]
- Bangsbo J, Gollnick PD, Graham TE, Juel C, Kiens B, Mizuno M, Saltin B: Anaerobic energy production and O2 deficit-debt relationship during exhaustive exercise in humans. J Physiol. 1990 Mar;422:539-59. [2352192 ]
- McCauley TG, Hamaguchi N, Stanton M: Aptamer-based biosensor arrays for detection and quantification of biological macromolecules. Anal Biochem. 2003 Aug 15;319(2):244-50. [12871718 ]
- Rush JW, MacLean DA, Hultman E, Graham TE: Exercise causes branched-chain oxoacid dehydrogenase dephosphorylation but not AMP deaminase binding. J Appl Physiol. 1995 Jun;78(6):2193-200. [7665417 ]
- McConell GK, Canny BJ, Daddo MC, Nance MJ, Snow RJ: Effect of carbohydrate ingestion on glucose kinetics and muscle metabolism during intense endurance exercise. J Appl Physiol. 2000 Nov;89(5):1690-8. [11053315 ]
- Swart PJ, Beljaars E, Smit C, Pasma A, Schuitemaker H, Meijer DK: Comparative pharmacokinetic, immunologic and hematologic studies on the anti-HIV-1/2 compounds aconitylated and succinylated HSA. J Drug Target. 1996;4(2):109-16. [8894971 ]
- Scott GS, Spitsin SV, Kean RB, Mikheeva T, Koprowski H, Hooper DC: Therapeutic intervention in experimental allergic encephalomyelitis by administration of uric acid precursors. Proc Natl Acad Sci U S A. 2002 Dec 10;99(25):16303-8. Epub 2002 Nov 25. [12451183 ]
|
|---|
| Synthesis Reference: |
Park, Yeong Hun; Cho, Gwang Myeong; Baek, Min Ji; Hong, Guk Gi; Lee, Jin Nam. Method for preparing 5'-inosinic acid by using microbe capable of over-expressing purC gene. Repub. Korea (2007), 7pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|