|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110462 |
|---|
|
Identification |
|---|
| Name: |
phosphoribulosylformimino-AICAR-P |
|---|
| Description: | An organophosphate oxoanion that is the tetraanionic form of 5-[(5-phospho-1-deoxy-D-ribulos-1-ylimino)methylamino]-1-(5-phospho-β-D-ribosyl)imidazole-4-carboxamide. |
|---|
|
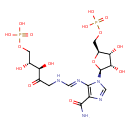
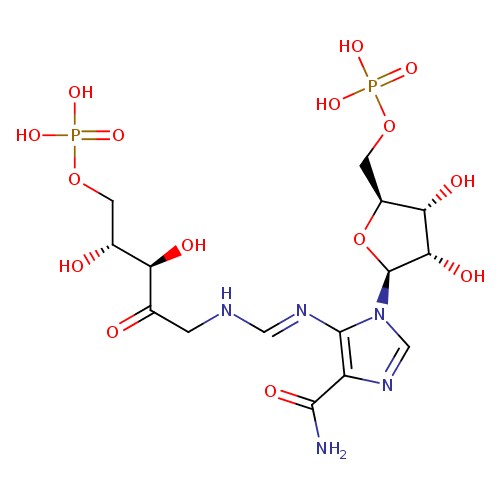
Structure |
|
|---|
| Synonyms: | -
5-[(5-phospho-1-deoxy-β-D-ribulos-1-ylamino)methylideneamino]-1-(5-phosphoribosyl)imidazole-4-carboxamide
-
N-(5'-phospho-D-1'-ribulosylformimino)-5-amino-1-(5''-phosphoribosyl)-4-imidazolecarboxamide
-
phosphoribulosylformiminoAICAR-phosphate
-
phosphoribulosyl-formimino-5-aminoimidazole-4-carboxamide ribotide phosphate
-
phosphoribulosyl-formimino-5-aminoimidazole-4-carboxamide ribonucleotide phosphate
-
PRFAR
-
N-(5'phospho-D-1'-ribulosylformimino)-5-amino-1-(5-phosphoribosyl)-4-imidazolecarboxamide
|
|---|
|
Chemical Formula: |
C15H21N5O15P2
|
|---|
| Average Molecular Weight: |
573.3 |
|---|
| Monoisotopic Molecular
Weight: |
577.08223818 |
|---|
| InChI Key: |
BLKFNHOCHNCLII-GHVQHMAVSA-J |
|---|
| InChI: |
InChI=1S/C15H25N5O15P2/c16-13(26)9-14(18-4-17-1-6(21)10(23)7(22)2-33-36(27,28)29)20(5-19-9)15-12(25)11(24)8(35-15)3-34-37(30,31)32/h4-5,7-8,10-12,15,22-25H,1-3H2,(H2,16,26)(H,17,18)(H2,27,28,29)(H2,30,31,32)/p-4/t7-,8-,10+,11-,12-,15-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 5- [(5- [(5- O- O- phosphonato- phosphonato- 1- 1- deoxy- deoxy- D- D- ribulos- ribulos- 1- 1- ylimino)methylamino]- ylimino)methylamino]- 1- 1- (5- (5- O- O- phosphonato- phosphonato- β- β- D- D- ribosyl)imidazole- ribosyl)imidazole- 4- 4- carboxamide carboxamide |
|---|
|
Traditional IUPAC Name: |
[(2R,3R)-5-[(E)-N'-{5-carbamoyl-3-[(2S,3S,4R,5S)-3,4-dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl]imidazol-4-yl}methenimidamido]-2,3-dihydroxy-4-oxopentyl]oxyphosphonic acid |
|---|
| SMILES: | C(OP(=O)([O-])[O-])C(O)C(O)C(=O)CN=CNC1(=C(N=CN1C2(C(O)C(C(COP(=O)([O-])[O-])O2)O))C(=O)N) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as 1-ribosyl-imidazolecarboxamides. These are organic compounds containing the imidazole ring linked to a ribose ring through a 1-2 bond. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Imidazole ribonucleosides and ribonucleotides |
|---|
|
Direct Parent |
1-ribosyl-imidazolecarboxamides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 1-ribosyl-imidazolecarboxamide
- Pentose-5-phosphate
- Pentose phosphate
- Glycosyl compound
- N-glycosyl compound
- Pentose monosaccharide
- Monosaccharide phosphate
- 2-heteroaryl carboxamide
- Monoalkyl phosphate
- Imidazole-4-carbonyl group
- Organic phosphoric acid derivative
- Alkyl phosphate
- Beta-hydroxy ketone
- Acyloin
- Phosphoric acid ester
- N-substituted imidazole
- Monosaccharide
- Alpha-hydroxy ketone
- Azole
- Imidazole
- Heteroaromatic compound
- Vinylogous amide
- Tetrahydrofuran
- Secondary alcohol
- Primary carboxylic acid amide
- Carboxamide group
- Ketone
- Oxacycle
- Organoheterocyclic compound
- Propargyl-type 1,3-dipolar organic compound
- Azacycle
- Organic 1,3-dipolar compound
- Amidine
- Carboxylic acid derivative
- Formamidine
- Carboxylic acid amidine
- Organooxygen compound
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Alcohol
- Organic nitrogen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- superpathway of histidine, purine, and pyrimidine biosynthesisPRPP-PWY
- L-histidine biosynthesisHISTSYN-PWY
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|