|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110461 |
|---|
|
Identification |
|---|
| Name: |
5-amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamide |
|---|
| Description: | An organophosphate oxoanion resulting from the removal of both protons from the phosphate group of 5-amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamide. It is the major species at pH 7.3. |
|---|
|
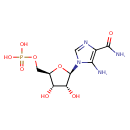
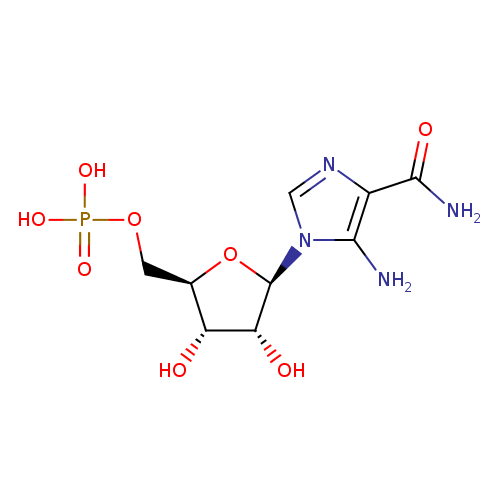
Structure |
|
|---|
| Synonyms: | -
Z-nucleotide
-
AICAR
-
AICA ribonucleotide
-
5'-phosphoribosyl-5-amino-4-imidazole carboxamide
-
5-amino-4-imidazolecarboxamide ribotide
-
5'-P-ribosyl-5-amino-4-imidazole carboxamide
-
aminoimidazole carboxamide ribonucleotide
|
|---|
|
Chemical Formula: |
C9H13N4O8P
|
|---|
| Average Molecular Weight: |
336.2 |
|---|
| Monoisotopic Molecular
Weight: |
338.0627499891 |
|---|
| InChI Key: |
NOTGFIUVDGNKRI-UUOKFMHZSA-L |
|---|
| InChI: |
InChI=1S/C9H15N4O8P/c10-7-4(8(11)16)12-2-13(7)9-6(15)5(14)3(21-9)1-20-22(17,18)19/h2-3,5-6,9,14-15H,1,10H2,(H2,11,16)(H2,17,18,19)/p-2/t3-,5-,6-,9-/m1/s1 |
|---|
| CAS
number: |
3031-94-5 |
|---|
| IUPAC Name: | 5- amino- amino- 1- 1- (5- (5- O- O- phosphonato- phosphonato- β- β- D- D- ribofuranosyl)- ribofuranosyl)- 1H- 1H- imidazole- imidazole- 4- 4- carboxamide carboxamide |
|---|
|
Traditional IUPAC Name: |
aica ribonucleotide |
|---|
| SMILES: | C(OP(=O)([O-])[O-])C1(C(O)C(O)C(O1)N2(C=NC(C(=O)N)=C(N)2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as 1-ribosyl-imidazolecarboxamides. These are organic compounds containing the imidazole ring linked to a ribose ring through a 1-2 bond. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Imidazole ribonucleosides and ribonucleotides |
|---|
|
Direct Parent |
1-ribosyl-imidazolecarboxamides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 1-ribosyl-imidazolecarboxamide
- Pentose-5-phosphate
- Pentose phosphate
- Glycosyl compound
- N-glycosyl compound
- Pentose monosaccharide
- Monosaccharide phosphate
- 2-heteroaryl carboxamide
- Imidazole-4-carbonyl group
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- N-substituted imidazole
- Alkyl phosphate
- Monosaccharide
- Aminoimidazole
- Primary aromatic amine
- Phosphoric acid ester
- Azole
- Imidazole
- Heteroaromatic compound
- Oxolane
- Vinylogous amide
- Primary carboxylic acid amide
- Secondary alcohol
- Amino acid or derivatives
- 1,2-diol
- Carboxamide group
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Carboxylic acid derivative
- Primary amine
- Organonitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Organic oxygen compound
- Amine
- Organic oxide
- Hydrocarbon derivative
- Organic nitrogen compound
- Alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE: 5'-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem. 2001 Dec 14;276(50):46912-6. Epub 2001 Oct 11. [11598104 ]
- Koistinen HA, Galuska D, Chibalin AV, Yang J, Zierath JR, Holman GD, Wallberg-Henriksson H: 5-amino-imidazole carboxamide riboside increases glucose transport and cell-surface GLUT4 content in skeletal muscle from subjects with type 2 diabetes. Diabetes. 2003 May;52(5):1066-72. [12716734 ]
- Koistinen HA, Chibalin AV, Zierath JR: Aberrant p38 mitogen-activated protein kinase signalling in skeletal muscle from Type 2 diabetic patients. Diabetologia. 2003 Oct;46(10):1324-8. Epub 2003 Aug 23. [12937895 ]
|
|---|
| Synthesis Reference: |
Schmitt, Laurent; Caperelli, Carol A. Enantiospecific synthesis of carbocyclic aminoimidazole carboxamide ribonucleotide (C-AICAR), succinoaminoimidazole carboxamide ribonucleotide (C-SAICAR), and a new intermediate for SAICAR analogs. Nucleosides & Nucleotides (1995), 14(9 & 10), 1929-45. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|