|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110459 |
|---|
|
Identification |
|---|
| Name: |
allantoate |
|---|
| Description: | A monocarboxylic acid anion that is the conjugate base of allantoic acid. |
|---|
|
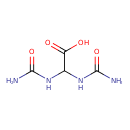
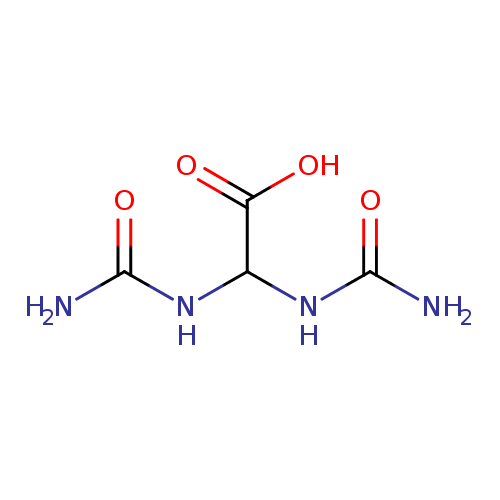
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C4H7N4O4
|
|---|
| Average Molecular Weight: |
175.12 |
|---|
| Monoisotopic Molecular
Weight: |
176.054554766 |
|---|
| InChI Key: |
NUCLJNSWZCHRKL-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/C4H8N4O4/c5-3(11)7-1(2(9)10)8-4(6)12/h1H,(H,9,10)(H3,5,7,11)(H3,6,8,12)/p-1 |
|---|
| CAS
number: |
99-16-1 |
|---|
| IUPAC Name: | bis(carbamoylamino)acetate |
|---|
|
Traditional IUPAC Name: |
allantoic acid |
|---|
| SMILES: | C(C(=O)[O-])(NC(=O)N)NC(=O)N |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as n-carbamoyl-alpha amino acids. These are compounds containing an alpha amino acid which bears an carbamoyl group at its terminal nitrogen atom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
N-carbamoyl-alpha amino acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- N-carbamoyl-alpha-amino acid
- Urea
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
180 - 181 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 180 - 181 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- allantoin degradation to ureidoglycolate I (urea producing)PWY-5697
- superpathway of allantoin degradation in plantsURDEGR-PWY
- allantoin degradation to glyoxylate IPWY-5694
- allantoin degradation to ureidoglycolate II (ammonia producing)PWY-5698
- allantoin degradation to glyoxylate IIIPWY-5705
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-0002-1900000000-21581f2d921374c16317 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-03di-9500000000-311fe8e2669139e60084 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-03k9-9000000000-eb594a4f54a39f5aaad4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-03di-9000000000-24cb204bd4d824cf2b53 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-003r-0900000000-91ad7fef4b504549f849 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-000i-9500000000-1f86fa2bb6e6a3e1988e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-000i-9000000000-6f90d802bf75ec4bc187 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-00dr-9000000000-3900e78a2acf61f39eff | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-00dl-9000000000-e98594c1e1b2aa50540a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Vigetti D, Pollegioni L, Monetti C, Prati M, Bernardini G, Gornati R: Property comparison of recombinant amphibian and mammalian allantoicases. FEBS Lett. 2002 Feb 13;512(1-3):323-8. [11852104 ]
|
|---|
| Synthesis Reference: |
Hermanowicz, Witold. Allantoic acid. Formation of allantoic acid from allantoin. Roczniki Chemii (1948), 22 159-80. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|