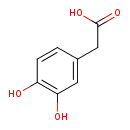
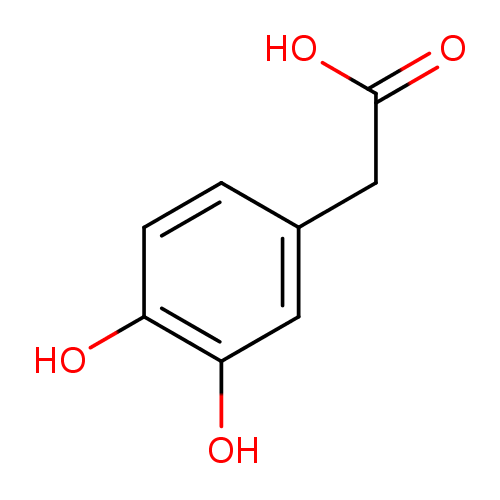
| References: |
- Goldstein DS, Eisenhofer G, Kopin IJ: Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharmacol Exp Ther. 2003 Jun;305(3):800-11. Epub 2003 Mar 20. [12649306 ]
- Panholzer TJ, Beyer J, Lichtwald K: Coupled-column liquid chromatographic analysis of catecholamines, serotonin, and metabolites in human urine. Clin Chem. 1999 Feb;45(2):262-8. [9931050 ]
- Raskind MA, Peskind ER, Holmes C, Goldstein DS: Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzheimer's disease. Biol Psychiatry. 1999 Sep 15;46(6):756-65. [10494443 ]
- Sjoberg S, Eriksson M, Nordin C: L-thyroxine treatment and neurotransmitter levels in the cerebrospinal fluid of hypothyroid patients: a pilot study. Eur J Endocrinol. 1998 Nov;139(5):493-7. [9849813 ]
- Eklundh T, Eriksson M, Sjoberg S, Nordin C: Monoamine precursors, transmitters and metabolites in cerebrospinal fluid: a prospective study in healthy male subjects. J Psychiatr Res. 1996 May-Jun;30(3):201-8. [8884658 ]
- Ebinger G, Michotte Y, Herregodts P: The significance of homovanillic acid and 3,4-dihydroxyphenylacetic acid concentrations in human lumbar cerebrospinal fluid. J Neurochem. 1987 Jun;48(6):1725-9. [3572399 ]
- Van Loon GR, De Souza EB, Kim C: Alterations in brain dopamine and serotonin metabolism during the development of tolerance to human beta-endorphin in rats. Can J Physiol Pharmacol. 1978 Dec;56(6):1067-71. [743624 ]
- Braestrup C: Biochemical differentiation of amphetamine vs methylphenidate and nomifensine in rats. J Pharm Pharmacol. 1977 Aug;29(8):463-70. [19594 ]
- Nakao N, Shintani-Mizushima A, Kakishita K, Itakura T: The ability of grafted human sympathetic neurons to synthesize and store dopamine: a potential mechanism for the clinical effect of sympathetic neuron autografts in patients with Parkinson's disease. Exp Neurol. 2004 Jul;188(1):65-73. [15191803 ]
- Annunziato LA, Wuerthele SM, Moore KE: Comparative effects of penfluridol on circling behavior and striatal DOPAC and serum prolactin concentrations in the rat. Eur J Pharmacol. 1978 Aug 1;50(3):187-92. [567584 ]
- De Simoni MG, Guardabasso V, Misterek K, Algeri S: Similarities and differences between D-ALA2 MET5 enkephalin amide and morphine in the induction of tolerance to their effects on catalepsy and on dopamine metabolism in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1982 Nov;321(2):105-11. [6891440 ]
- Gramsch C, Blasig J, Herz A: Changes in striatal dopamine metabolism during precipitated morphine withdrawal. Eur J Pharmacol. 1977 Aug 1;44(3):231-40. [560969 ]
- Fornstedt B, Brun A, Rosengren E, Carlsson A: The apparent autoxidation rate of catechols in dopamine-rich regions of human brains increases with the degree of depigmentation of substantia nigra. J Neural Transm Park Dis Dement Sect. 1989;1(4):279-95. [2597314 ]
- Garrett MC, Soares-da-Silva P: Increased cerebrospinal fluid dopamine and 3,4-dihydroxyphenylacetic acid levels in Huntington's disease: evidence for an overactive dopaminergic brain transmission. J Neurochem. 1992 Jan;58(1):101-6. [1309230 ]
- Massotti M, Longo VG: Role of the dopaminergic system in the cataleptogenic action of bulbocapnine. J Pharm Pharmacol. 1979 Oct;31(10):691-5. [41042 ]
- Tekes K, Tothfalusi L, Gaal J, Magyar K: Effect of MAO inhibitors on the uptake and metabolism of dopamine in rat and human brain. Pol J Pharmacol Pharm. 1988 Nov-Dec;40(6):653-8. [3152003 ]
- Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, Fang Y, Larson JL, McDougall JA, Chester JA, Saez C, Pugsley TA, Gershanik O, Low MJ, Grandy DK: Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell. 1997 Sep 19;90(6):991-1001. [9323127 ]
- Hutson PH, Curzon G: Dopamine metabolites in rat cisternal cerebrospinal fluid: major contribution from extrastriatal dopamine neurones. J Neurochem. 1986 Jan;46(1):186-90. [2415677 ]
- Thurmond JB, Brown JW: Effect of brain monoamine precursors on stress-induced behavioral and neurochemical changes in aged mice. Brain Res. 1984 Mar 26;296(1):93-102. [6201238 ]
- Kogan BM, Tkachenko AA, Drozdov AZ, Andrianova EP, Filatova TS, Man'kovskaia IV, Kovaleva IA: [Monoamine metabolism in different forms of paraphilias] Zh Nevropatol Psikhiatr Im S S Korsakova. 1995;95(6):52-6. [8788979 ]
- Florang VR, Rees JN, Brogden NK, Anderson DG, Hurley TD, Doorn JA: Inhibition of the oxidative metabolism of 3,4-dihydroxyphenylacetaldehyde, a reactive intermediate of dopamine metabolism, by 4-hydroxy-2-nonenal. Neurotoxicology. 2007 Jan;28(1):76-82. Epub 2006 Aug 1. [16956664 ]
- Jiang H, Jiang Q, Liu W, Feng J: Parkin suppresses the expression of monoamine oxidases. J Biol Chem. 2006 Mar 31;281(13):8591-9. Epub 2006 Feb 2. [16455660 ]
- Cadet JL, Ali SF, Rothman RB, Epstein CJ: Neurotoxicity, drugs and abuse, and the CuZn-superoxide dismutase transgenic mice. Mol Neurobiol. 1995 Aug-Dec;11(1-3):155-63. [8561959 ]
- Pestana M, Jardim H, Correia F, Vieira-Coelho MA, Soares-da-Silva P: Renal dopaminergic mechanisms in renal parenchymal diseases and hypertension. Nephrol Dial Transplant. 2001;16 Suppl 1:53-9. [11369822 ]
- Kim DH, Kim SY, Park SY, Han MJ: Metabolism of quercitrin by human intestinal bacteria and its relation to some biological activities. Biol Pharm Bull. 1999 Jul;22(7):749-51. [10443478 ]
- Gao K, Xu A, Krul C, Venema K, Liu Y, Niu Y, Lu J, Bensoussan L, Seeram NP, Heber D, Henning SM: Of the major phenolic acids formed during human microbial fermentation of tea, citrus, and soy flavonoid supplements, only 3,4-dihydroxyphenylacetic acid has antiproliferative activity. J Nutr. 2006 Jan;136(1):52-7. [16365058 ]
|
|---|