|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110435 |
|---|
|
Identification |
|---|
| Name: |
(R)-S-lactoylglutathione |
|---|
| Description: | The S-[(R)-lactoyl] derivative of glutathione. It is an intermediate in the pyruvate metabolism. |
|---|
|
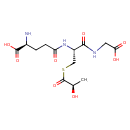
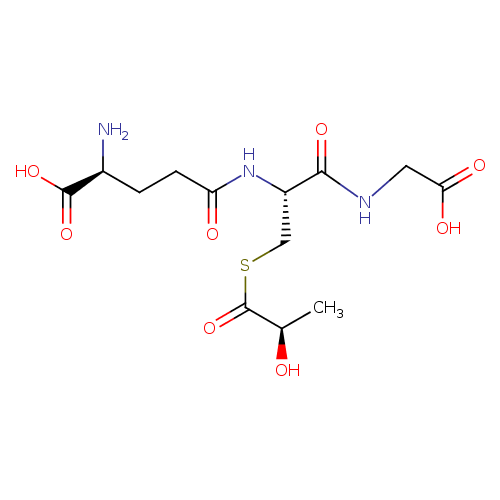
Structure |
|
|---|
| Synonyms: | -
S-D-lactoylglutathione
-
D-lactoylglutathione
|
|---|
|
Chemical Formula: |
C13H20N3O8S
|
|---|
| Average Molecular Weight: |
378.38 |
|---|
| Monoisotopic Molecular
Weight: |
380.1127603886 |
|---|
| InChI Key: |
VDYDCVUWILIYQF-CSMHCCOUSA-M |
|---|
| InChI: |
InChI=1S/C13H21N3O8S/c1-6(17)13(24)25-5-8(11(21)15-4-10(19)20)16-9(18)3-2-7(14)12(22)23/h6-8,17H,2-5,14H2,1H3,(H,15,21)(H,16,18)(H,19,20)(H,22,23)/p-1/t6-,7+,8+/m1/s1 |
|---|
| CAS
number: |
25138-66-3 |
|---|
| IUPAC Name: | S- [(2R)- [(2R)- 2- 2- hydroxypropanoyl]- hydroxypropanoyl]- γ- γ- L- L- glutamyl- glutamyl- L- L- cysteinylglycine cysteinylglycine |
|---|
|
Traditional IUPAC Name: |
S-lactoylglutathione |
|---|
| SMILES: | CC(O)C(=O)SCC(C(NCC([O-])=O)=O)NC(=O)CCC([N+])C([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Oligopeptides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-oligopeptide
- Gamma-glutamyl alpha peptide
- S-(2-hydroxyacyl)glutathione
- S-acylglutathione
- Glutamine or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Alpha-amino acid amide
- Cysteine or derivatives
- Alpha-amino acid
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- L-alpha-amino acid
- N-acyl-amine
- Fatty acyl
- Fatty amide
- Dicarboxylic acid or derivatives
- Fatty acid
- Secondary carboxylic acid amide
- Secondary alcohol
- Amino acid or derivatives
- Thiocarboxylic acid ester
- Amino acid
- Carboxamide group
- Carbothioic s-ester
- Sulfenyl compound
- Carboxylic acid
- Thiocarboxylic acid or derivatives
- Organosulfur compound
- Primary amine
- Organic nitrogen compound
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Amine
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Primary aliphatic amine
- Organooxygen compound
- Organonitrogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Inoue Y, Rhee H, Watanabe K, Murata K, Kimura A (1987)Metabolism of 2-ketoaldehydes in mold: purification and characterization of glyoxalase I from Aspergillus niger. Journal of biochemistry 102, Pubmed: 3123469
- Liu Y, Hama H, Fujita Y, Kondo A, Inoue Y, Kimura A, Fukuda H (1999)Production of S-lactoylglutathione by high activity whole cell biocatalysts prepared by permeabilization of recombinant saccharomyces cerevisiae with alcohols Biotechnology and bioengineering 64, Pubmed: 10397839
|
|---|
| Synthesis Reference: |
Liu, Yan; Hama, Hideki; Fujita, Yasuya; Kondo, Akihiko; Inoue, Yoshiharu; Kimura, Akira; Fu Production of S-lactoylglutathione by high activity whole cell biocatalysts prepared by permeabilization of recombinant Saccharomyces cerevisiae with alcohols. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|