|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110429 |
|---|
|
Identification |
|---|
| Name: |
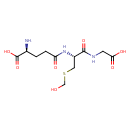
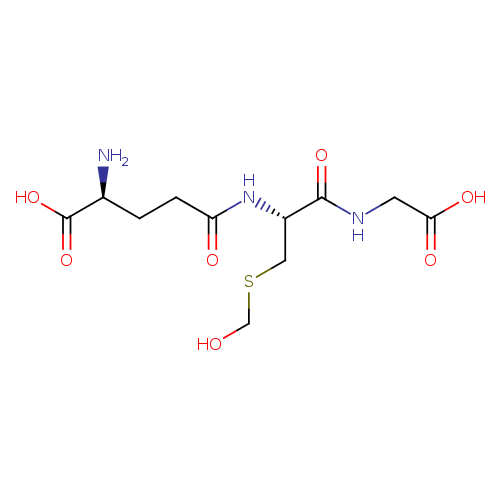
S-hydroxymethylglutathione |
|---|
| Description: | Conjugate base of S-(hydroxymethyl)glutathione. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
glycine
- N-(N-γ-L-S-(hydroxymethyl)-L-cysteinyl)-
-
HMGSH
-
HM-GSH
-
GS-CH2-OH
-
hydroxymethylglutathione
|
|---|
|
Chemical Formula: |
C11H18N3O7S
|
|---|
| Average Molecular Weight: |
338.1021957023 |
|---|
| Monoisotopic Molecular
Weight: |
338.1021957023 |
|---|
| InChI Key: |
PIUSLWSYOYFRFR-BQBZGAKWSA-M |
|---|
| InChI: |
InChI=1S/C11H19N3O7S/c12-6(11(20)21)1-2-8(16)14-7(4-22-5-15)10(19)13-3-9(17)18/h6-7,15H,1-5,12H2,(H,13,19)(H,14,16)(H,17,18)(H,20,21)/p-1/t6-,7-/m0/s1 |
|---|
| CAS
number: |
32260-87-0 |
|---|
| IUPAC Name: | (2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)carbamoyl]-2-[(hydroxymethyl)sulfanyl]ethyl]carbamoyl}butanoic acid |
|---|
|
Traditional IUPAC Name: |
S-hydroxymethylglutathione |
|---|
| SMILES: | C(NC(=O)C(CSCO)NC(=O)CCC([N+])C(=O)[O-])C(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Oligopeptides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-oligopeptide
- Gamma-glutamyl alpha peptide
- Glutamine or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- Cysteine or derivatives
- Alpha-amino acid
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- L-alpha-amino acid
- Dicarboxylic acid or derivatives
- Fatty amide
- Fatty acyl
- Fatty acid
- N-acyl-amine
- Amino acid or derivatives
- Carboxamide group
- Amino acid
- Secondary carboxylic acid amide
- Sulfenyl compound
- Carboxylic acid
- Organopnictogen compound
- Primary aliphatic amine
- Hydrocarbon derivative
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Amine
- Organic oxide
- Primary amine
- Organosulfur compound
- Organonitrogen compound
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Holmquist B, Moulis JM, Engeland K, Vallee BL: Role of arginine 115 in fatty acid activation and formaldehyde dehydrogenase activity of human class III alcohol dehydrogenase. Biochemistry. 1993 May 18;32(19):5139-44. [8494891 ]
- Yang ZN, Bosron WF, Hurley TD: Structure of human chi chi alcohol dehydrogenase: a glutathione-dependent formaldehyde dehydrogenase. J Mol Biol. 1997 Jan 24;265(3):330-43. [9018047 ]
- Koivusalo M, Baumann M, Uotila L: Evidence for the identity of glutathione-dependent formaldehyde dehydrogenase and class III alcohol dehydrogenase. FEBS Lett. 1989 Oct 23;257(1):105-9. [2806555 ]
- Sanghani PC, Stone CL, Ray BD, Pindel EV, Hurley TD, Bosron WF: Kinetic mechanism of human glutathione-dependent formaldehyde dehydrogenase. Biochemistry. 2000 Sep 5;39(35):10720-9. [10978156 ]
- Lee SL, Wang MF, Lee AI, Yin SJ: The metabolic role of human ADH3 functioning as ethanol dehydrogenase. FEBS Lett. 2003 Jun 5;544(1-3):143-7. [12782305 ]
- Gutheil WG, Holmquist B, Vallee BL: Purification, characterization, and partial sequence of the glutathione-dependent formaldehyde dehydrogenase from Escherichia coli: a class III alcohol dehydrogenase. Biochemistry. 1992 Jan 21;31(2):475-81. [1731906 ]
- Danielsson O, Shafqat J, Estonius M, el-Ahmad M, Jornvall H: Isozyme multiplicity with anomalous dimer patterns in a class III alcohol dehydrogenase. Effects on the activity and quaternary structure of residue exchanges at "nonfunctional" sites in a native protein. Biochemistry. 1996 Nov 19;35(46):14561-8. [8931553 ]
- Sanghani PC, Bosron WF, Hurley TD: Human glutathione-dependent formaldehyde dehydrogenase. Structural changes associated with ternary complex formation. Biochemistry. 2002 Dec 24;41(51):15189-94. [12484756 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|