|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110410 |
|---|
|
Identification |
|---|
| Name: |
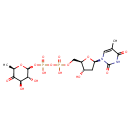
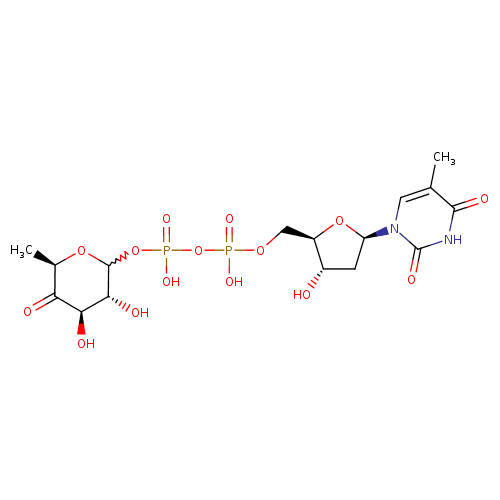
dTDP-4-dehydro-6-deoxy-α-D-glucopyranose |
|---|
| Description: | Dianion of dTDP-4-dehydro-6-deoxy-α-D-glucose arising from deprotonation of the diphosphate OH groups; major species at pH 7.3. It is an intermediate in dTDP-rhamnose biosynthesis. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
TDP-4-keto-6-deoxy--α-D-glucose
-
TDP-4-oxo-6-deoxy--α-D-glucose
-
dTDP-4-oxo-6-deoxy--α-D-glucose
-
dTDP-6-deoxy-α-D-xylo-4-hexosulose
-
dTDP-6-deoxy-α-D-xylohex-4-ulose
-
dTDP-4-dehydro-6-deoxy-α-D-glucose
|
|---|
|
Chemical Formula: |
C16H22N2O15P2
|
|---|
| Average Molecular Weight: |
544.3 |
|---|
| Monoisotopic Molecular
Weight: |
546.0651911323 |
|---|
| InChI Key: |
PSXWNITXWWECNY-UCBTUHGZSA-L |
|---|
| InChI: |
InChI=1S/C16H24N2O15P2/c1-6-4-18(16(24)17-14(6)23)10-3-8(19)9(31-10)5-29-34(25,26)33-35(27,28)32-15-13(22)12(21)11(20)7(2)30-15/h4,7-10,12-13,15,19,21-22H,3,5H2,1-2H3,(H,25,26)(H,27,28)(H,17,23,24)/p-2/t7-,8+,9-,10-,12+,13-,15-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | thymidine 5'-[3-(6-deoxy-α-D-xylo-hexopyranosyl-4-ulose) diphosphate] |
|---|
|
Traditional IUPAC Name: |
[(3R,4R,6R)-3,4-dihydroxy-6-methyl-5-oxooxan-2-yl]oxy({hydroxy[(2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxo-3H-pyrimidin-1-yl)oxolan-2-yl]methoxyphosphoryl}oxy)phosphinic acid |
|---|
| SMILES: | CC1(=CN(C(=O)NC(=O)1)C3(CC(O)C(COP(=O)([O-])OP(=O)([O-])OC2(OC(C)C(=O)C(O)C(O)2))O3)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pyrimidine nucleotide sugars. These are pyrimidine nucleotides bound to a saccharide derivative through the terminal phosphate group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Pyrimidine nucleotides |
|---|
|
Direct Parent |
Pyrimidine nucleotide sugars |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrimidine nucleotide sugar
- Pyrimidine 2'-deoxyribonucleoside diphosphate
- Pentose phosphate
- Organic pyrophosphate
- Pyrimidone
- Monoalkyl phosphate
- Hydropyrimidine
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Oxane
- Pyrimidine
- Alkyl phosphate
- Heteroaromatic compound
- Oxolane
- Vinylogous amide
- Ketone
- Lactam
- Secondary alcohol
- Urea
- Cyclic ketone
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Alcohol
- Carbonyl group
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Ma Y, Mills JA, Belisle JT, Vissa V, Howell M, Bowlin K, Scherman MS, McNeil M (1997)Determination of the pathway for rhamnose biosynthesis in mycobacteria: cloning, sequencing and expression of the Mycobacterium tuberculosis gene encoding alpha-D-glucose-1-phosphate thymidylyltransferase. Microbiology (Reading, England) 143 ( Pt 3), Pubmed: 9084178
- Ma Y, Stern RJ, Scherman MS, Vissa VD, Yan W, Jones VC, Zhang F, Franzblau SG, Lewis WH, McNeil MR (2001)Drug targeting Mycobacterium tuberculosis cell wall synthesis: genetics of dTDP-rhamnose synthetic enzymes and development of a microtiter plate-based screen for inhibitors of conversion of dTDP-glucose to dTDP-rhamnose. Antimicrobial agents and chemotherapy 45, Pubmed: 11302803
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|