|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110405 |
|---|
|
Identification |
|---|
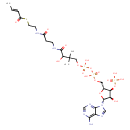
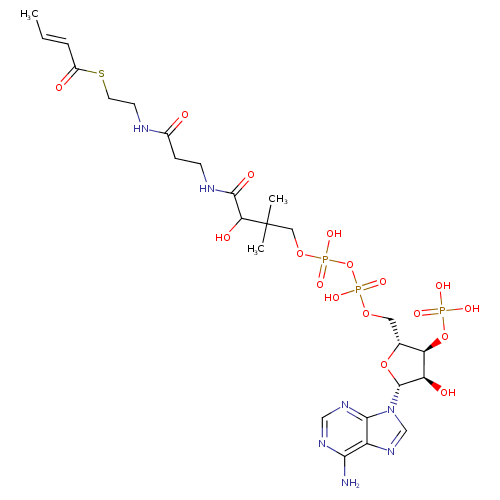
| Name: |
crotonyl-CoA |
|---|
| Description: | Tetraanion of crotonoyl-CoA arising from deprotonation of phosphate and diphosphate functions. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
crotonyl-S-CoA
-
crotonyl-coenzyme A
-
crotonoyl-CoA
-
2-butenoyl-CoA
-
trans-but-2-enoyl-CoA
-
but-2-enoyl-CoA
-
trans-butyr-2-enoyl-CoA
-
(E)-but-2-enoyl-CoA
|
|---|
|
Chemical Formula: |
C25H36N7O17P3S
|
|---|
| Average Molecular Weight: |
831.58 |
|---|
| Monoisotopic Molecular
Weight: |
835.1414231161 |
|---|
| InChI Key: |
KFWWCMJSYSSPSK-BOGFJHSMSA-J |
|---|
| InChI: |
InChI=1S/C25H40N7O17P3S/c1-4-5-16(34)53-9-8-27-15(33)6-7-28-23(37)20(36)25(2,3)11-46-52(43,44)49-51(41,42)45-10-14-19(48-50(38,39)40)18(35)24(47-14)32-13-31-17-21(26)29-12-30-22(17)32/h4-5,12-14,18-20,24,35-36H,6-11H2,1-3H3,(H,27,33)(H,28,37)(H,41,42)(H,43,44)(H2,26,29,30)(H2,38,39,40)/p-4/b5-4+/t14-,18-,19-,20?,24-/m1/s1 |
|---|
| CAS
number: |
102680-35-3 |
|---|
| IUPAC Name: | 3'- phosphonatoadenosine 5'- phosphonatoadenosine 5'- {3- {3- [(3R)- [(3R)- 4- 4- ({3- ({3- [(2- [(2- {[(2E)- {[(2E)- but- but- 2- 2- enoyl]sulfanyl}ethyl)amino]- enoyl]sulfanyl}ethyl)amino]- 3- 3- oxopropyl}amino)- oxopropyl}amino)- 3- 3- hydroxy- hydroxy- 2,2- 2,2- dimethyl- dimethyl- 4- 4- oxobutyl] diphosphate} oxobutyl] diphosphate} |
|---|
|
Traditional IUPAC Name: |
[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-2-({[(3-{[2-({2-[(2E)-but-2-enoylsulfanyl]ethyl}carbamoyl)ethyl]carbamoyl}-3-hydroxy-2,2-dimethylpropoxy(hydroxy)phosphoryl)oxy(hydroxy)phosphoryl]oxy}methyl)-4-hydroxyoxolan-3-yl]oxyphosphonic acid |
|---|
| SMILES: | CC=CC(SCCNC(CCNC(C(C(COP(=O)([O-])OP(OCC1(OC(C(C1OP([O-])([O-])=O)O)N3(C=NC2(C(=NC=NC=23)N))))([O-])=O)(C)C)O)=O)=O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as 2-enoyl coas. These are organic compounds containing a coenzyme A substructure linked to a 2-enoyl chain. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Lipids and lipid-like molecules |
|---|
| Sub Class | Fatty Acyls |
|---|
|
Direct Parent |
2-enoyl CoAs |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Pentose phosphate
- Pentose-5-phosphate
- Ribonucleoside 3'-phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Organic pyrophosphate
- 6-aminopurine
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Aminopyrimidine
- Fatty amide
- Imidolactam
- Monosaccharide
- N-acyl-amine
- N-substituted imidazole
- Organic phosphoric acid derivative
- Alkyl phosphate
- Phosphoric acid ester
- Primary aromatic amine
- Pyrimidine
- Oxolane
- Azole
- Imidazole
- Heteroaromatic compound
- Amino acid or derivatives
- Thiocarboxylic acid ester
- Carboxamide group
- Carbothioic s-ester
- Secondary carboxylic acid amide
- Secondary alcohol
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Carboxylic acid derivative
- Hydrocarbon derivative
- Alcohol
- Organic nitrogen compound
- Amine
- Organonitrogen compound
- Carbonyl group
- Organooxygen compound
- Organosulfur compound
- Organic oxygen compound
- Organopnictogen compound
- Primary amine
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Fu Z, Wang M, Paschke R, Rao KS, Frerman FE, Kim JJ: Crystal structures of human glutaryl-CoA dehydrogenase with and without an alternate substrate: structural bases of dehydrogenation and decarboxylation reactions. Biochemistry. 2004 Aug 3;43(30):9674-84. [15274622 ]
- Hyman DB, Tanaka K: Specific glutaryl-CoA dehydrogenating activity is deficient in cultured fibroblasts from glutaric aciduria patients. J Clin Invest. 1984 Mar;73(3):778-84. [6423663 ]
- Kalousek F, Darigo MD, Rosenberg LE: Isolation and characterization of propionyl-CoA carboxylase from normal human liver. Evidence for a protomeric tetramer of nonidentical subunits. J Biol Chem. 1980 Jan 10;255(1):60-5. [6765947 ]
- Dwyer TM, Rao KS, Westover JB, Kim JJ, Frerman FE: The function of Arg-94 in the oxidation and decarboxylation of glutaryl-CoA by human glutaryl-CoA dehydrogenase. J Biol Chem. 2001 Jan 5;276(1):133-8. [11024031 ]
- Babidge W, Millard S, Roediger W: Sulfides impair short chain fatty acid beta-oxidation at acyl-CoA dehydrogenase level in colonocytes: implications for ulcerative colitis. Mol Cell Biochem. 1998 Apr;181(1-2):117-24. [9562248 ]
- Lenich AC, Goodman SI: The purification and characterization of glutaryl-coenzyme A dehydrogenase from porcine and human liver. J Biol Chem. 1986 Mar 25;261(9):4090-6. [3081514 ]
- Gregersen N, Brandt NJ, Christensen E, Gron I, Rasmussen K, Brandt S: Glutaric aciduria: clinical and laboratory findings in two brothers. J Pediatr. 1977 May;90(5):740-5. [853337 ]
- Hodgins MB: Possible mechanisms of androgen resistance in 5 alpha-reductase deficiency: implications for the physiological roles of 5 alpha-reductases. J Steroid Biochem. 1983 Jul;19(1B):555-9. [6887883 ]
- Saenger AK, Nguyen TV, Vockley J, Stankovich MT: Thermodynamic regulation of human short-chain acyl-CoA dehydrogenase by substrate and product binding. Biochemistry. 2005 Dec 13;44(49):16043-53. [16331964 ]
- Finocchiaro G, Ito M, Tanaka K: Purification and properties of short chain acyl-CoA, medium chain acyl-CoA, and isovaleryl-CoA dehydrogenases from human liver. J Biol Chem. 1987 Jun 15;262(17):7982-9. [3597357 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|