|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110388 |
|---|
|
Identification |
|---|
| Name: |
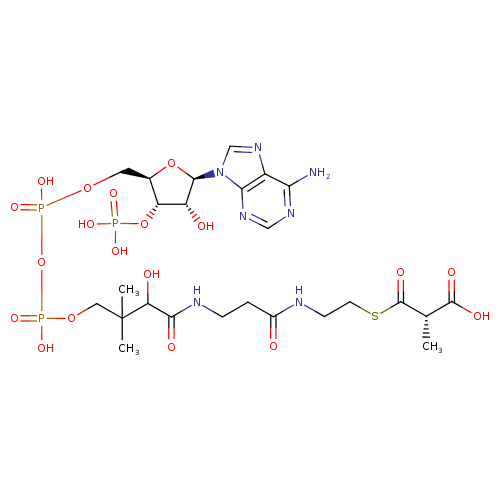
(S)-methylmalonyl-CoA |
|---|
| Description: | Pentaanion of (S)-methylmalonyl-CoA arising from deprotonation of phosphate, diphosphate and carboxylic acid functions. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
D-methylmalonyl-CoA
-
methyl-malonyl-coenzyme A
-
CH3-malonyl-CoA
-
(2S)-methyl-malonyl-CoA
|
|---|
|
Chemical Formula: |
C25H35N7O19P3S
|
|---|
| Average Molecular Weight: |
862.57 |
|---|
| Monoisotopic Molecular
Weight: |
867.1312523603 |
|---|
| InChI Key: |
MZFOKIKEPGUZEN-IBNUZSNCSA-I |
|---|
| InChI: |
InChI=1S/C25H40N7O19P3S/c1-12(23(37)38)24(39)55-7-6-27-14(33)4-5-28-21(36)18(35)25(2,3)9-48-54(45,46)51-53(43,44)47-8-13-17(50-52(40,41)42)16(34)22(49-13)32-11-31-15-19(26)29-10-30-20(15)32/h10-13,16-18,22,34-35H,4-9H2,1-3H3,(H,27,33)(H,28,36)(H,37,38)(H,43,44)(H,45,46)(H2,26,29,30)(H2,40,41,42)/p-5/t12-,13+,16+,17+,18-,22+/m0/s1 |
|---|
| CAS
number: |
104809-02-1 |
|---|
| IUPAC Name: | (2S)-3-{[2-(3-{3-[({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)methyl]-2-hydroxy-3-methylbutanamido}propanamido)ethyl]sulfanyl}-2-methyl-3-oxopropanoic acid |
|---|
|
Traditional IUPAC Name: |
(2S)-3-({2-[3-(3-{[({[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]methyl}-2-hydroxy-3-methylbutanamido)propanamido]ethyl}sulfanyl)-2-methyl-3-oxopropanoic acid |
|---|
| SMILES: | CC(C(=O)SCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(=O)(OP(=O)(OCC1(C(OP([O-])(=O)[O-])C(O)C(O1)N3(C2(=C(C(N)=NC=N2)N=C3))))[O-])[O-])C([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as acyl coas. These are organic compounds containing a coenzyme A substructure linked to an acyl chain. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Lipids and lipid-like molecules |
|---|
| Sub Class | Fatty Acyls |
|---|
|
Direct Parent |
Acyl CoAs |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Pentose phosphate
- Pentose-5-phosphate
- Ribonucleoside 3'-phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Organic pyrophosphate
- 6-aminopurine
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Aminopyrimidine
- Fatty amide
- Imidolactam
- Monosaccharide
- N-acyl-amine
- N-substituted imidazole
- Organic phosphoric acid derivative
- Alkyl phosphate
- 1,3-dicarbonyl compound
- Phosphoric acid ester
- Primary aromatic amine
- Pyrimidine
- Oxolane
- Azole
- Imidazole
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Secondary alcohol
- Thiocarboxylic acid ester
- Carboxamide group
- Carbothioic s-ester
- Amino acid
- Oxacycle
- Organoheterocyclic compound
- Azacycle
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organic nitrogen compound
- Alcohol
- Amine
- Organonitrogen compound
- Carbonyl group
- Organooxygen compound
- Organosulfur compound
- Organic oxygen compound
- Organopnictogen compound
- Primary amine
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -5 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Valine, Leucine and Isoleucine Degradation pae00280
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Watkins D, Rosenblatt DS: Inborn errors of cobalamin absorption and metabolism. Am J Med Genet C Semin Med Genet. 2011 Feb 15;157C(1):33-44. doi: 10.1002/ajmg.c.30288. Epub 2011 Feb 10. [21312325 ]
- Quadros EV: Advances in the understanding of cobalamin assimilation and metabolism. Br J Haematol. 2010 Jan;148(2):195-204. doi: 10.1111/j.1365-2141.2009.07937.x. Epub 2009 Oct 12. [19832808 ]
- Rincon A, Aguado C, Desviat LR, Sanchez-Alcudia R, Ugarte M, Perez B: Propionic and methylmalonic acidemia: antisense therapeutics for intronic variations causing aberrantly spliced messenger RNA. Am J Hum Genet. 2007 Dec;81(6):1262-70. [17966092 ]
- Morrow G 3rd, Barness LA, Cardinale GJ, Abeles RH, Flaks JG: Congenital methylmalonic acidemia: enzymatic evidence for two forms of the disease. Proc Natl Acad Sci U S A. 1969 May;63(1):191-7. [5257962 ]
- Loewy AD, Niles KM, Anastasio N, Watkins D, Lavoie J, Lerner-Ellis JP, Pastinen T, Trasler JM, Rosenblatt DS: Epigenetic modification of the gene for the vitamin B(12) chaperone MMACHC can result in increased tumorigenicity and methionine dependence. Mol Genet Metab. 2009 Apr;96(4):261-7. doi: 10.1016/j.ymgme.2008.12.011. Epub 2009 Feb 5. [19200761 ]
- Ludwig ML, Matthews RG: Structure-based perspectives on B12-dependent enzymes. Annu Rev Biochem. 1997;66:269-313. [9242908 ]
- Shevell MI, Matiaszuk N, Ledley FD, Rosenblatt DS: Varying neurological phenotypes among muto and mut- patients with methylmalonylCoA mutase deficiency. Am J Med Genet. 1993 Mar 1;45(5):619-24. [7681251 ]
- Gu P, Welch WH, Guo L, Schegg KM, Blomquist GJ: Characterization of a novel microsomal fatty acid synthetase (FAS) compared to a cytosolic FAS in the housefly, Musca domestica. Comp Biochem Physiol B Biochem Mol Biol. 1997 Oct;118(2):447-56. [9440236 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|