|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110385 |
|---|
|
Identification |
|---|
| Name: |
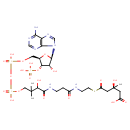
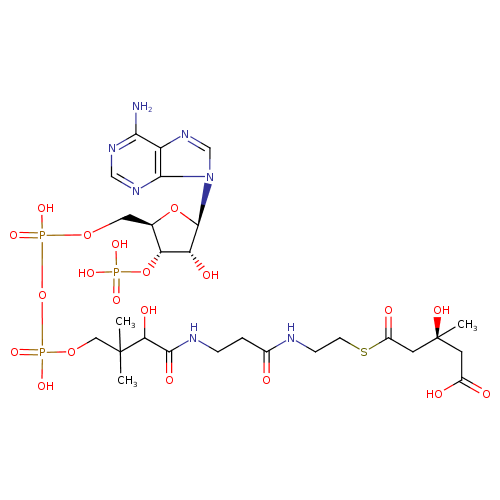
(S)-3-hydroxy-3-methylglutaryl-CoA |
|---|
| Description: | An acyl-CoA oxoanion that results from the removal of all five protons from the phosphate and carboxylic acid groups of (3S)-3-hydroxy-3-methylglutaryl-CoA. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
3-hydroxy-3-methyl-glutaryl-CoA
-
HMG-CoA
-
hydroxymethylglutaryl-CoA
-
3-hydroxy-3-methylglutaryl-coenzyme A
-
3-Hydroxy-3-methylglutaryl-CoA
|
|---|
|
Chemical Formula: |
C27H39N7O20P3S
|
|---|
| Average Molecular Weight: |
906.62 |
|---|
| Monoisotopic Molecular
Weight: |
911.1574671108 |
|---|
| InChI Key: |
CABVTRNMFUVUDM-VRHQGPGLSA-I |
|---|
| InChI: |
InChI=1S/C27H44N7O20P3S/c1-26(2,21(40)24(41)30-5-4-15(35)29-6-7-58-17(38)9-27(3,42)8-16(36)37)11-51-57(48,49)54-56(46,47)50-10-14-20(53-55(43,44)45)19(39)25(52-14)34-13-33-18-22(28)31-12-32-23(18)34/h12-14,19-21,25,39-40,42H,4-11H2,1-3H3,(H,29,35)(H,30,41)(H,36,37)(H,46,47)(H,48,49)(H2,28,31,32)(H2,43,44,45)/p-5/t14-,19-,20-,21+,25-,27+/m1/s1 |
|---|
| CAS
number: |
1553-55-5 |
|---|
| IUPAC Name: | (3S)-5-{[2-(3-{3-[({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)methyl]-2-hydroxy-3-methylbutanamido}propanamido)ethyl]sulfanyl}-3-hydroxy-3-methyl-5-oxopentanoic acid |
|---|
|
Traditional IUPAC Name: |
(3S)-5-({2-[3-(3-{[({[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]methyl}-2-hydroxy-3-methylbutanamido)propanamido]ethyl}sulfanyl)-3-hydroxy-3-methyl-5-oxopentanoic acid |
|---|
| SMILES: | CC(C)(C(O)C(=O)NCCC(=O)NCCSC(=O)CC(C)(O)CC(=O)[O-])COP(=O)(OP(=O)(OCC1(C(OP([O-])(=O)[O-])C(O)C(O1)N3(C2(=C(C(N)=NC=N2)N=C3))))[O-])[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as 3-hydroxy-3-alkylglutaryl coas. These are alpha, omega dicarboxyacyl-CoA that result from the formal condensation of the thiol group of coenzyme A with one of the carboxy groups of 3-hydroxy-3-alkylglutaric acid. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Lipids and lipid-like molecules |
|---|
| Sub Class | Fatty Acyls |
|---|
|
Direct Parent |
3-hydroxy-3-alkylglutaryl CoAs |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Pentose phosphate
- Pentose-5-phosphate
- Ribonucleoside 3'-phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Organic pyrophosphate
- 6-aminopurine
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Aminopyrimidine
- Fatty amide
- Imidolactam
- Monosaccharide
- N-acyl-amine
- N-substituted imidazole
- Organic phosphoric acid derivative
- Alkyl phosphate
- Phosphoric acid ester
- Primary aromatic amine
- Pyrimidine
- Tertiary alcohol
- Heteroaromatic compound
- Imidazole
- Azole
- Oxolane
- Carboxamide group
- Carbothioic s-ester
- Secondary alcohol
- Amino acid
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Thiocarboxylic acid ester
- Organoheterocyclic compound
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Oxacycle
- Thiocarboxylic acid or derivatives
- Sulfenyl compound
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organic nitrogen compound
- Alcohol
- Amine
- Organonitrogen compound
- Carbonyl group
- Organooxygen compound
- Organosulfur compound
- Organic oxygen compound
- Organopnictogen compound
- Primary amine
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -5 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Sato T, Oouchi M, Nagakubo H, Chiba T, Ogawa S, Sato C, Sugimura K, Fukuda M: Effect of pravastatin on plasma ketone bodies in diabetics with hypercholesterolemia. Tohoku J Exp Med. 1998 May;185(1):25-9. [9710942 ]
- Wysocki SJ, Wilkinson SP, Hahnel R, Wong CY, Panegyres PK: 3-Hydroxy-3-methylglutaric aciduria, combined with 3-methylglutaconic aciduria. Clin Chim Acta. 1976 Aug 2;70(3):399-406. [947633 ]
- Lange Y, Ye J, Steck TL: Activation of membrane cholesterol by displacement from phospholipids. J Biol Chem. 2005 Oct 28;280(43):36126-31. Epub 2005 Aug 29. [16129675 ]
- Li X, Liu L, Tupper JC, Bannerman DD, Winn RK, Sebti SM, Hamilton AD, Harlan JM: Inhibition of protein geranylgeranylation and RhoA/RhoA kinase pathway induces apoptosis in human endothelial cells. J Biol Chem. 2002 May 3;277(18):15309-16. Epub 2002 Feb 11. [11839765 ]
- Mital BK, Garg SK: Anticarcinogenic, hypocholesterolemic, and antagonistic activities of Lactobacillus acidophilus. Crit Rev Microbiol. 1995;21(3):175-214. [8845062 ]
- Nozaki S, Nakagawa T, Nakata A, Yamashita S, Kameda-Takemura K, Nakamura T, Keno Y, Tokunaga K, Matsuzawa Y: Effects of pravastatin on plasma and urinary mevalonate concentrations in subjects with familial hypercholesterolaemia: a comparison of morning and evening administration. Eur J Clin Pharmacol. 1996;49(5):361-4. [8866629 ]
- Ubels FL, Muntinga JH, van Doormaal JJ, Reitsma WD, Smit AJ: Effects of initial and long-term lipid-lowering therapy on vascular wall characteristics. Atherosclerosis. 2001 Jan;154(1):155-61. [11137095 ]
- Huang L, Wang Y, Grimm S: ATP-dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein. Drug Metab Dispos. 2006 May;34(5):738-42. Epub 2006 Jan 13. [16415124 ]
- Son BK, Kozaki K, Iijima K, Eto M, Kojima T, Ota H, Senda Y, Maemura K, Nakano T, Akishita M, Ouchi Y: Statins protect human aortic smooth muscle cells from inorganic phosphate-induced calcification by restoring Gas6-Axl survival pathway. Circ Res. 2006 Apr 28;98(8):1024-31. Epub 2006 Mar 23. [16556867 ]
- Gianni L, Di Padova F, Zuin M, Podda M: Bile acid-induced inhibition of the lymphoproliferative response to phytohemagglutinin and pokeweed mitogen: an in vitro study. Gastroenterology. 1980 Feb;78(2):231-5. [7350045 ]
- Jenke HS, Lowel M, Berndt J: Effect of alterations in vitro and in vivo of the cholesterol content in rat liver microsomes on the activity of 3-hydroxy-3-methylglutaryl-CoA reductase. Hoppe Seylers Z Physiol Chem. 1983 Feb;364(2):135-40. [6840702 ]
- Naseem SM, Heald FP: Sex mediated lipid metabolism in human aortic smooth muscle cells. Biochem Biophys Res Commun. 1987 Apr 14;144(1):284-91. [3579907 ]
- Knight BL, Patel DD, Soutar AK: The regulation of 3-hydroxy-3-methylglutaryl-CoA reductase activity, cholesterol esterification and the expression of low-density lipoprotein receptors in cultured monocyte-derived macrophages. Biochem J. 1983 Feb 15;210(2):523-32. [6305342 ]
- Martin J, Denver R, Bailey M, Krum H: In vitro inhibitory effects of atorvastatin on cardiac fibroblasts: implications for ventricular remodelling. Clin Exp Pharmacol Physiol. 2005 Sep;32(9):697-701. [16173924 ]
- Plotkin D, Miller S, Nakajima S, Peskin E, Burkman R, Richardson D, Mitchel Y, Waldstreicher J, Liu M, Shapiro D, Santoro N: Lowering low density lipoprotein cholesterol with simvastatin, a hydroxy-3-methylglutaryl-coenzyme a reductase inhibitor, does not affect luteal function in premenopausal women. J Clin Endocrinol Metab. 2002 Jul;87(7):3155-61. [12107216 ]
- Gil G, Smith JR, Goldstein JL, Brown MS: Optional exon in the 5'-untranslated region of 3-hydroxy-3-methylglutaryl coenzyme A synthase gene: conserved sequence and splicing pattern in humans and hamsters. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1863-6. [3470763 ]
- Freeman MR, Solomon KR: Cholesterol and prostate cancer. J Cell Biochem. 2004 Jan 1;91(1):54-69. [14689582 ]
|
|---|
| Synthesis Reference: |
Williamson I P; Rodwell V W Isolation and purification of 3-hydroxy-3-methylglutaryl-coenzyme A by ion-exchange chromatography. Journal of lipid research (1981), 22(1), 184-7. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|