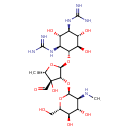
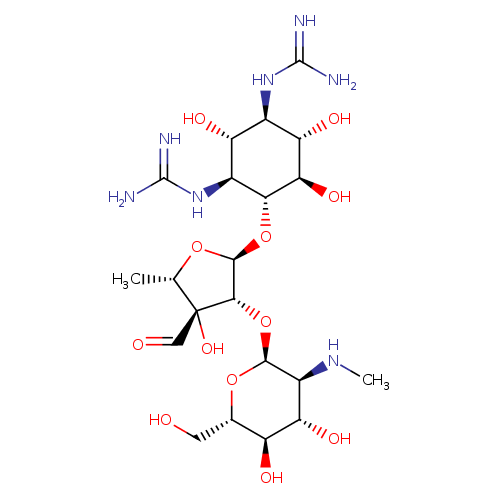
streptomycin (PAMDB110370)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB110370 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | streptomycin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Trication of streptomycin arising from protonation of the guanidino and secondary amino groups. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: | • 2,4-diguanidino-3,5,6-Trihydroxycyclohexyl 5-deoxy-2-O-(2-deoxy-2-methylamino-alpha-L-glucopyranosyl)-3-C-formyl-beta-L-lyxopentanofuranoside • [2-Deoxy-2-(dimethylamino)-alpha-L-glucopyranosyl]-(1>2)-[5-deoxy-3-C-formyl-alpha-L-lyxofuranosyl]-(1>4)-{n',n'''-[(1,3,5/2,4,6)-2,4,5,6-tetrahydroxycyclohexane-1,3-diyl]diguanidine} • Kantrex • SM • Streomycin • 2,4-diguanidino-3,5,6-Trihydroxycyclohexyl 5-deoxy-2-O-(2-deoxy-2-methylamino-a-L-glucopyranosyl)-3-C-formyl-b-L-lyxopentanofuranoside • 2,4-diguanidino-3,5,6-Trihydroxycyclohexyl 5-deoxy-2-O-(2-deoxy-2-methylamino-?-L-glucopyranosyl)-3-C-formyl-?-L-lyxopentanofuranoside • [2-Deoxy-2-(dimethylamino)-a-L-glucopyranosyl]-(1>2)-[5-deoxy-3-C-formyl-a-L-lyxofuranosyl]-(1>4)-{n',n'''-[(1,3,5/2,4,6)-2,4,5,6-tetrahydroxycyclohexane-1,3-diyl]diguanidine} • [2-Deoxy-2-(dimethylamino)-?-L-glucopyranosyl]-(1>2)-[5-deoxy-3-C-formyl-?-L-lyxofuranosyl]-(1>4)-{n',n'''-[(1,3,5/2,4,6)-2,4,5,6-tetrahydroxycyclohexane-1,3-diyl]diguanidine} • Streptomycin a sulfate • Streptomycin sesquisulfate hydrate • Streptomycin sesquisulphate hydrate • Streptomycin sulfate • Streptomycin sulphate • Streptomycin, sulfate salt • Normon brand OF streptomycin sulfate • Sanavita brand OF streptomycin sulfate • strepto Hefa • strepto-Fatol • Streptomycin gr?nenthal • Wernigerode brand OF streptomycin sulfate • Clariana brand OF streptomycin sulfate • Estreptomicina cepa • Fatol brand OF streptomycin sulfate • strepto Fatol • strepto-Hefa • CEPA brand OF streptomycin sulfate • Estreptomicina normon • Gr?nenthal brand OF streptomycin sulfate • Panpharma brand OF streptomycin sulfate • Estreptomicina clariana • Streptomycin sulfate (2:3) salt • Streptomycine panpharma | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C21H42N7O12 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 584.6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 584.2891448498 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | UCSJYZPVAKXKNQ-HZYVHMACSA-Q | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C21H39N7O12/c1-5-21(36,4-30)16(40-17-9(26-2)13(34)10(31)6(3-29)38-17)18(37-5)39-15-8(28-20(24)25)11(32)7(27-19(22)23)12(33)14(15)35/h4-18,26,29,31-36H,3H2,1-2H3,(H4,22,23,27)(H4,24,25,28)/p+3/t5-,6-,7+,8-,9-,10-,11+,12-,13-,14+,15+,16-,17-,18-,21+/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 57-92-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (1R,2R,3S,4R,5R,6S)- | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | streptomycin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC1(OC(C(C1(C=O)O)OC2(C(C(C(C(O2)CO)O)O)[N+]C))OC3(C(C(C(C(C3O)O)NC(N)=[N+])O)NC(N)=[N+])) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as aminocyclitol glycosides. These are organic compounds containing an amicocyclitol moiety glycosidically linked to a carbohydrate moiety. There are two major classes of aminoglycosides containing a 2-streptamine core. They are called 4,5- and 4,6-disubstituted 2-deoxystreptamines. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic oxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organooxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Carbohydrates and carbohydrate conjugates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Aminocyclitol glycosides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | +3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||