|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110254 |
|---|
|
Identification |
|---|
| Name: |
guanylyl molybdenum cofactor |
|---|
| Description: | An organophosphate oxoanion arising from deprotonation of the diphosphate OH groups of Mo(VI)-molybdopterin guanine dinucleotide. |
|---|
|
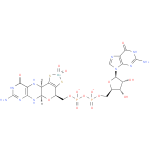
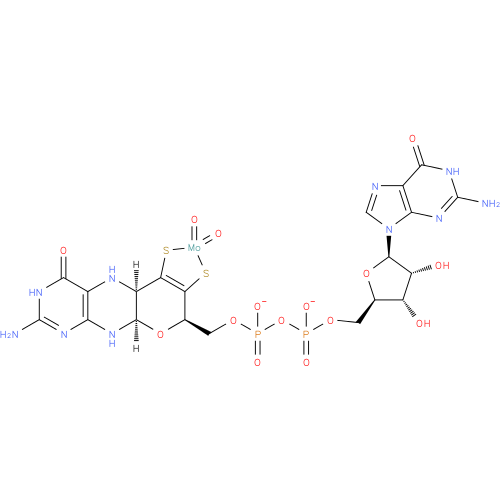
Structure |
|
|---|
| Synonyms: | -
MGD
-
MPT-GMP
-
MoO2(OH)Dtpp-mGDP
-
molybdopterin guanine dinucleotide
|
|---|
|
Chemical Formula: |
C20H22N10O15P2S2Mo
|
|---|
| Average Molecular Weight: |
864.46 |
|---|
| Monoisotopic Molecular
Weight: |
867.9393323539 |
|---|
| InChI Key: |
RQPREYSMTBNTJA-JNXJJIQFSA-J |
|---|
| InChI: |
InChI=1S/C20H26N10O13P2S2.Mo.2O/c21-19-26-13-7(15(33)28-19)24-6-12(47)11(46)5(41-17(6)25-13)2-40-45(37,38)43-44(35,36)39-1-4-9(31)10(32)18(42-4)30-3-23-8-14(30)27-20(22)29-16(8)34;;;/h3-6,9-10,17-18,24,31-32,46-47H,1-2H2,(H,35,36)(H,37,38)(H3,22,27,29,34)(H4,21,25,26,28,33);;;/q;+2;;/p-4/t4-,5-,6+,9-,10-,17-,18-;;;/m1.../s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | {5'- O- O- [{[{[(5aR,8R,9aR)- [{[{[(5aR,8R,9aR)- 2- 2- amino- amino- 4- 4- oxo- oxo- 6,7- 6,7- di(sulfanyl- di(sulfanyl- κS)- κS)- 3,5,5a,8,9a,10- 3,5,5a,8,9a,10- hexahydro- hexahydro- 4H- 4H- pyrano[3,2- pyrano[3,2- g]pteridin- g]pteridin- 8- 8- yl]methoxy}(hydroxy)phosphoryl]oxy}(hydroxy)phosphoryl]guanosinato(4−)}(dioxo)molybdate(2−) yl]methoxy}(hydroxy)phosphoryl]oxy}(hydroxy)phosphoryl]guanosinato(4−)}(dioxo)molybdate(2−) |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | C(OP(=O)([O-])OP(=O)([O-])OCC3(O[CH]1(NC4(N=C(N)NC(=O)C(N[CH]1C2(=C(S[Mo](=O)(=O)S2)3))=4))))C5(OC(C(O)C(O)5)N7(C=NC6(C(=O)NC(N)=NC=67))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as molybdopterin dinucleotides. These are a dinucleotide that is made up of a molybdopterin and a purine or pyrimidine base linked to each other through a phosphate chain. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
|
Class |
Molybdopterin dinucleotides |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Molybdopterin dinucleotides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Molybdopterin dinucleotide
- Purine ribonucleoside diphosphate
- Molybdopterin
- Purine ribonucleoside monophosphate
- Pyranopterin
- Pentose phosphate
- Pentose-5-phosphate
- Pterin
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- 6-oxopurine
- Organic pyrophosphate
- Pentose monosaccharide
- Hypoxanthine
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- N-substituted imidazole
- Imidolactam
- Monosaccharide
- Alkyl phosphate
- Pyran
- Pyrimidine
- Vinylogous amide
- Heteroaromatic compound
- Azole
- Imidazole
- Tetrahydrofuran
- Lactam
- 1,2-diol
- Secondary alcohol
- Oxacycle
- Azacycle
- Organic metal salt
- Organic transition metal salt
- Organoheterocyclic compound
- Metalloheterocycle
- Secondary amine
- Organic oxygen compound
- Organic salt
- Hydrocarbon derivative
- Amine
- Alcohol
- Organic oxide
- Organopnictogen compound
- Organic nitrogen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic anion
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
| Property | Value | Source |
|---|
| Molecular Weight | 864.469 g/mol | PubChem | | Hydrogen Bond Donor Count | 8 | PubChem | | Hydrogen Bond Acceptor Count | 20 | PubChem | | Rotatable Bond Count | 9 | PubChem | | Exact Mass | 865.924 g/mol | PubChem | | Monoisotopic Mass | 865.924 g/mol | PubChem | | Topological Polar Surface Area | 380 A^2 | PubChem | | Heavy Atom Count | 50 | PubChem | | Formal Charge | -2 | PubChem | | Complexity | 1660 | PubChem | | Isotope Atom Count | 0 | PubChem | | Defined Atom Stereocenter Count | 4 | PubChem | | Undefined Atom Stereocenter Count | 3 | PubChem | | Defined Bond Stereocenter Count | 0 | PubChem | | Undefined Bond Stereocenter Count | 0 | PubChem | | Covalently-Bonded Unit Count | 2 | PubChem |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|