|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110213 |
|---|
|
Identification |
|---|
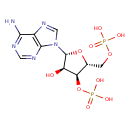
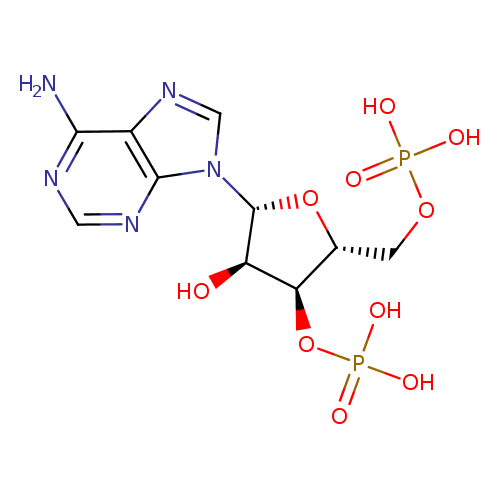
| Name: |
adenosine 3',5'-bisphosphate |
|---|
| Description: | An organophosphate oxoanion arising from deprotonation of the phosphate OH groups of adenosine 3',5'-bismonophosphate; major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
adenosine-3',5'-bisphosphate
-
3',5'-ADP
-
phosphoadenosine phosphate
-
PAP
|
|---|
|
Chemical Formula: |
C10H11N5O10P2
|
|---|
| Average Molecular Weight: |
423.17 |
|---|
| Monoisotopic Molecular
Weight: |
427.0294147485 |
|---|
| InChI Key: |
WHTCPDAXWFLDIH-KQYNXXCUSA-J |
|---|
| InChI: |
InChI=1S/C10H15N5O10P2/c11-8-5-9(13-2-12-8)15(3-14-5)10-6(16)7(25-27(20,21)22)4(24-10)1-23-26(17,18)19/h2-4,6-7,10,16H,1H2,(H2,11,12,13)(H2,17,18,19)(H2,20,21,22)/p-4/t4-,6-,7-,10-/m1/s1 |
|---|
| CAS
number: |
1053-73-2 |
|---|
| IUPAC Name: | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}phosphonic acid |
|---|
|
Traditional IUPAC Name: |
adenosine 3',5'-bisphosphate |
|---|
| SMILES: | C(OP(=O)([O-])[O-])C1(C(OP(=O)([O-])[O-])C(O)C(O1)N3(C=NC2(C(N)=NC=NC=23))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as purine ribonucleoside 3',5'-bisphosphates. These are purine ribobucleotides with one phosphate group attached to 3' and 5' hydroxyl groups of the ribose moiety. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
Purine ribonucleoside 3',5'-bisphosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside monophosphate
- Ribonucleoside 3'-phosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Monoalkyl phosphate
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Primary aromatic amine
- Imidolactam
- Alkyl phosphate
- Pyrimidine
- Oxolane
- Azole
- Imidazole
- Heteroaromatic compound
- Secondary alcohol
- Azacycle
- Oxacycle
- Organoheterocyclic compound
- Organic oxide
- Organic nitrogen compound
- Hydrocarbon derivative
- Alcohol
- Amine
- Organic oxygen compound
- Primary amine
- Organonitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 614.5 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Gasmi L, McLennan AG: The mouse Nudt7 gene encodes a peroxisomal nudix hydrolase specific for coenzyme A and its derivatives. Biochem J. 2001 Jul 1;357(Pt 1):33-8. [11415433 ]
- Lewis AJ, Otake Y, Walle UK, Walle T: Sulphonation of N-hydroxy-2-acetylaminofluorene by human dehydroepiandrosterone sulphotransferase. Xenobiotica. 2000 Mar;30(3):253-61. [10752640 ]
- Leonidas DD, Chavali GB, Oikonomakos NG, Chrysina ED, Kosmopoulou MN, Vlassi M, Frankling C, Acharya KR: High-resolution crystal structures of ribonuclease A complexed with adenylic and uridylic nucleotide inhibitors. Implications for structure-based design of ribonucleolytic inhibitors. Protein Sci. 2003 Nov;12(11):2559-74. [14573867 ]
- Turner NA, Moake JL, McIntire LV: Blockade of adenosine diphosphate receptors P2Y(12) and P2Y(1) is required to inhibit platelet aggregation in whole blood under flow. Blood. 2001 Dec 1;98(12):3340-5. [11719372 ]
- Traut TW: Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994 Nov 9;140(1):1-22. [7877593 ]
- Russo N, Acharya KR, Vallee BL, Shapiro R: A combined kinetic and modeling study of the catalytic center subsites of human angiogenin. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):804-8. [8570639 ]
|
|---|
| Synthesis Reference: |
Tsunako, Mitsutomo; Kotone, Akira. Preparation of nucleoside-2',5'- and 3',5'-diphosphoric acids. Jpn. Kokai Tokkyo Koho (1991), 13 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|