|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110210 |
|---|
|
Identification |
|---|
| Name: |
(R)-1-aminopropan-2-ol |
|---|
| Description: | An ammonium ion obtained by protonation of the amino group of (2R)-1-aminopropan-2-ol. |
|---|
|
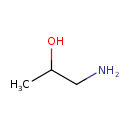
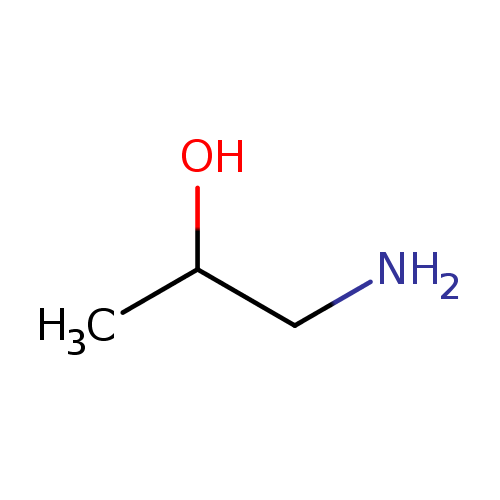
Structure |
|
|---|
| Synonyms: | -
(R)-1-aminopropanol
-
D-1-amino-2-propanol
-
D-1-aminopropan-2-ol
|
|---|
|
Chemical Formula: |
C3H10NO
|
|---|
| Average Molecular Weight: |
76.118 |
|---|
| Monoisotopic Molecular
Weight: |
76.0762389483 |
|---|
| InChI Key: |
HXKKHQJGJAFBHI-GSVOUGTGSA-O |
|---|
| InChI: |
InChI=1S/C3H9NO/c1-3(5)2-4/h3,5H,2,4H2,1H3/p+1/t3-/m1/s1 |
|---|
| CAS
number: |
78-96-6 |
|---|
| IUPAC Name: | (2R)-2-hydroxypropan-1-aminium |
|---|
|
Traditional IUPAC Name: |
1-amino-2-propanol |
|---|
| SMILES: | CC(O)C[N+] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as 1,2-aminoalcohols. These are organic compounds containing an alkyl chain with an amine group bound to the C1 atom and an alcohol group bound to the C2 atom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic nitrogen compounds |
|---|
| Sub Class | Organonitrogen compounds |
|---|
|
Direct Parent |
1,2-aminoalcohols |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Secondary alcohol
- 1,2-aminoalcohol
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Primary aliphatic amine
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | +1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Peru KM, Headley JV, Doucette WJ: Determination of alkanolamines in cattails (Typha latifolia) utilizing electrospray ionization with selected reaction monitoring and ion-exchange chromatography. Rapid Commun Mass Spectrom. 2004;18(14):1629-34. [15282789 ]
- Hervin RL, Lucas JB: Occupational health case report. No. 8. Monoisopropanolamine. J Occup Med. 1974 May;16(5):355-7. [4274990 ]
- Saghir SA, Frantz SW, Spence MW, Nolan RJ, Lowe ER, Rick DL, Bartels MJ: Pharmacokinetics and bioavailability of diisopropanolamine (DIPA) in rats following intravenous or dermal application. Food Chem Toxicol. 2007 Oct;45(10):2047-56. Epub 2007 May 18. [17583405 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|