|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110198 |
|---|
|
Identification |
|---|
| Name: |
3-methyl-2-oxobutanoate |
|---|
| Description: | A 2-oxo monocarboxylic acid anion that is the conjugate base of 3-methyl-2-oxobutanoic acid, arising from deprotonation of the carboxy group. |
|---|
|
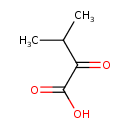
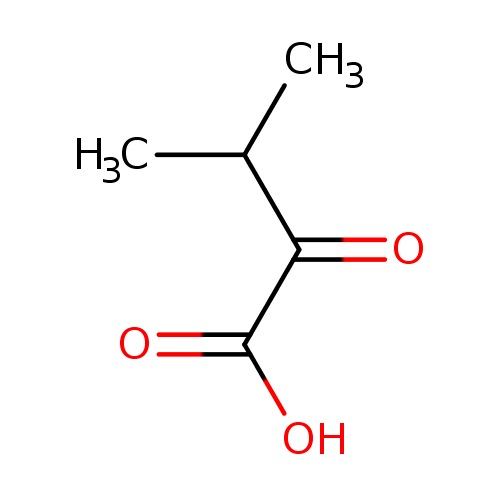
Structure |
|
|---|
| Synonyms: | -
2-oxo-3-methylbutanoate
-
2-oxoisovalerate
-
α-keto-isovaleric acid
-
α-ketoisopentanoic acid
-
α-keto-isovalerate
-
α-oxoisovalerate
-
α-ketovaline
-
2-keto-isovalerate
-
2-ketovaline
-
α-keto-valine
-
2-oxoisopentanoate
-
2-keto-3-methylbutyric acid
|
|---|
|
Chemical Formula: |
C5H7O3
|
|---|
| Average Molecular Weight: |
115.11 |
|---|
| Monoisotopic Molecular
Weight: |
116.0473441231 |
|---|
| InChI Key: |
QHKABHOOEWYVLI-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/C5H8O3/c1-3(2)4(6)5(7)8/h3H,1-2H3,(H,7,8)/p-1 |
|---|
| CAS
number: |
759-05-7 |
|---|
| IUPAC Name: | 3-methyl-2-oxobutanoate |
|---|
|
Traditional IUPAC Name: |
?-ketoisovalerate |
|---|
| SMILES: | CC(C(C([O-])=O)=O)C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as short-chain keto acids and derivatives. These are keto acids with an alkyl chain the contains less than 6 carbon atoms. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Keto acids and derivatives |
|---|
|
Direct Parent |
Short-chain keto acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Branched fatty acid
- Methyl-branched fatty acid
- Short-chain keto acid
- Alpha-keto acid
- Fatty acyl
- Alpha-hydroxy ketone
- Ketone
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Carbonyl group
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
31.5 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 31.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Valine, Leucine and Isoleucine Degradation pae00280
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-000i-9500000000-ff936b879a69b5d118f8 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-000i-8920000000-e37b37d64d43dcf763f0 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 1 TMS) | splash10-000i-9400000000-e3995acc4818a98d0f48 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 1 TMS) | splash10-0f79-9720000000-5d89487273e44ea61a68 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-000i-9000000000-10ab58a33e9ca7dbace0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-000i-9000000000-ad51ff01c94b6046ad64 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-014i-0900000000-9993174a7b1801b90ddb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-00xr-9500000000-293818b81e0879b6feb2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-00di-9000000000-75058f27a2178b9cf121 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-0006-9000000000-4c20af39e8ee009d5278 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00r2-9400000000-21f8c3fae79161c82099 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-9000000000-5f25e41413738bb6b5d7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-801af00dea93fcfd637d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01b9-8900000000-5185c7dfc72c25069904 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00xs-9200000000-7e2a275a65197f96f5d7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0600-9000000000-0013e0ff06f9896a1337 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Dickinson JR, Harrison SJ, Hewlins MJ (1998)An investigation of the metabolism of valine to isobutyl alcohol in Saccharomyces cerevisiae. The Journal of biological chemistry 273, Pubmed: 9748245
|
|---|
| Synthesis Reference: |
Pirrung, Michael C.; Ha, Hyun Joon; Holmes, Christopher P. Purification and inhibition of spinach a,b-dihydroxyacid dehydratase . Journal of Organic Chemistry (1989), 54(7), 1543-8. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|