|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110167 |
|---|
|
Identification |
|---|
| Name: |
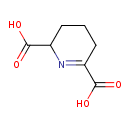
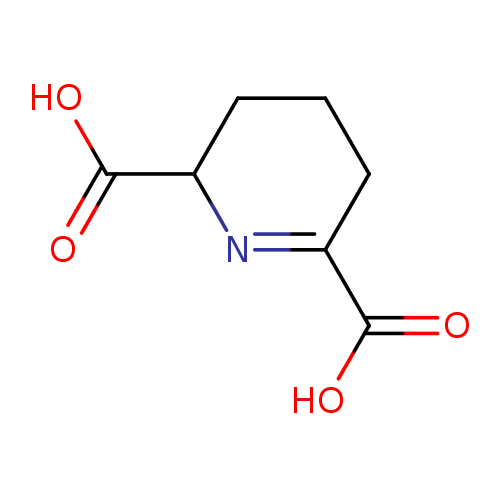
(S)-2,3,4,5-tetrahydrodipicolinate |
|---|
| Description: | Tetrahydrodipicolinate, converted from L-aspartate, is an important intermediate in lysine biosynthesis pathway. Several pathways are now recognized in bacteria, most algae, fungi and higher plants for the biosynthesis of lysine. They are divided into two groups - the diaminopimelate (DAP) pathways, and the α-aminoadipate (AAA) pathways. In the pathways that belong to the DAP group, lysine is produced from aspartate (along with methionine, threonine and isoleucine). All of these pathways share the upper segments, which include the four steps required for conversion of L-aspartate to tetrahydrodipicolinate. They also share the last step, which is the conversion of the intermediate meso-diaminopimelate (D,L-DAP, or meso-DAP) to lysine. However, these pathways differ in the routes leading from tetrahydrodipicolinate to meso-diaminopimelate. The four variations include: (I) the succinylase variant, which involves succinylated intermediates. In this route tetrahydrodipicolinate is coverted to meso-diaminopimelate in four enzymatic steps; (II) the acetylase variant, which involves acetylated intermediates. This route also involves four enzymatic steps for the conversion of tetrahydrodipicolinate to meso-diaminopimelate; (III) the dehydrogenase variant, in which tetrahydrodipicolinate is converted to meso-diaminopimelate in a single enzymatic step; (IV) the diaminopimelate-aminotransferase variant, in which tetrahydrodipicolinate is converted to meso-diaminopimelate in two steps. In addition to lysine, the pathways in this group also produce meso-DAP, which is an important metabolite on its own. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
2,3,4,5-tetrahydrodipicolinate
-
(S)-2,3,4,5-tetrahydropyridine-2,6-dicarboxylate
-
Δ1-piperideine-2,6-dicarboxylate
-
tetrahydrodipicolinate
-
tetrahydropyridine-2,6-dicarboxylate
-
L-2,3,4,5-tetrahydrodipicolinate
|
|---|
|
Chemical Formula: |
C7H7NO4
|
|---|
| Average Molecular Weight: |
169.14 |
|---|
| Monoisotopic Molecular
Weight: |
171.0531577825 |
|---|
| InChI Key: |
CXMBCXQHOXUCEO-BYPYZUCNSA-L |
|---|
| InChI: |
InChI=1S/C7H9NO4/c9-6(10)4-2-1-3-5(8-4)7(11)12/h4H,1-3H2,(H,9,10)(H,11,12)/p-2/t4-/m0/s1 |
|---|
| CAS
number: |
2353-17-5 |
|---|
| IUPAC Name: | (2S)-2,3,4,5-tetrahydropyridine-2,6-dicarboxylate |
|---|
|
Traditional IUPAC Name: |
thdpa |
|---|
| SMILES: | C1(CC(=NC(C1)C([O-])=O)C([O-])=O) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as alpha amino acids and derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon), or a derivative thereof. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Alpha amino acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-amino acid or derivatives
- Tetrahydropyridine
- Dicarboxylic acid or derivatives
- Hydropyridine
- Ketimine
- Azacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboxylic acid
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Imine
- Organic nitrogen compound
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- superpathway of L-lysine, L-threonine and L-methionine biosynthesis IP4-PWY
- L-lysine biosynthesis IDAPLYSINESYN-PWY
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|