|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110137 |
|---|
|
Identification |
|---|
| Name: |
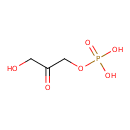
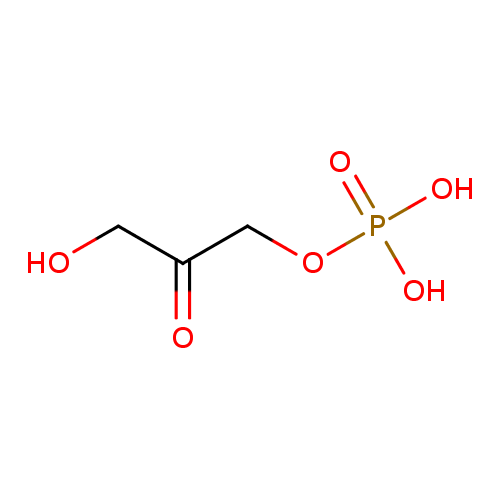
glycerone phosphate |
|---|
| Description: | A dianionic form of glycerone phosphate arising from deprotonation of the phosphate OH groups; major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
DHAP
-
dihydroxyacetone phosphate
-
dihydroxyacetone-P
-
di-OH-acetone-P
-
dihydroxy-acetone phosphate
-
3-hydroxy-2-oxopropyl phosphate
|
|---|
|
Chemical Formula: |
C3H5O6P
|
|---|
| Average Molecular Weight: |
168.04 |
|---|
| Monoisotopic Molecular
Weight: |
169.9980244673 |
|---|
| InChI Key: |
GNGACRATGGDKBX-UHFFFAOYSA-L |
|---|
| InChI: |
InChI=1S/C3H7O6P/c4-1-3(5)2-9-10(6,7)8/h4H,1-2H2,(H2,6,7,8)/p-2 |
|---|
| CAS
number: |
57-04-5 |
|---|
| IUPAC Name: | 3-hydroxy-2-oxopropyl phosphate |
|---|
|
Traditional IUPAC Name: |
dihydroxyacetone-phosphate |
|---|
| SMILES: | C(C(=O)CO)OP([O-])([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as monosaccharide phosphates. These are monosaccharides comprising a phosphated group linked to the carbohydrate unit. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Monosaccharide phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Glycerone phosphate
- Monosaccharide phosphate
- Monoalkyl phosphate
- Alkyl phosphate
- Glycerone or derivatives
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Alpha-hydroxy ketone
- Ketone
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 3 TMS) | splash10-0uy4-3954100000-ab5b096e0eac9831cf42 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 3 TMS) | splash10-0g0m-3964100000-cd938c4cea382029ce88 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-7900000000-fec8dd084ee59efa2286 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0kmi-9800000000-beb639c3fffc815897cc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4j-9000000000-580658c1ae323fa4f718 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-016r-6900000000-4a0e75761c8eb8071d1e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9100000000-7e5e1e00ef8f891ffe3e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-08f155d8875692abc94b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-7900000000-fec8dd084ee59efa2286 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0kmi-9800000000-beb639c3fffc815897cc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4j-9000000000-580658c1ae323fa4f718 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-016r-6900000000-4a0e75761c8eb8071d1e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9100000000-7e5e1e00ef8f891ffe3e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-08f155d8875692abc94b | View in MoNA |
|---|
|
|---|
|
References |
|---|
| References: |
- Nakayama Y, Kinoshita A, Tomita M: Dynamic simulation of red blood cell metabolism and its application to the analysis of a pathological condition. Theor Biol Med Model. 2005 May 9;2(1):18. [15882454 ]
- Roberts NB, Dutton J, Helliwell T, Rothwell PJ, Kavanagh JP: Pyrophosphate in synovial fluid and urine and its relationship to urinary risk factors for stone disease. Ann Clin Biochem. 1992 Sep;29 ( Pt 5):529-34. [1332571 ]
- Yamamoto T, Moriwaki Y, Takahashi S, Ohata H, Nakano T, Yamakita J, Higashino K: Effect of glucagon on the xylitol-induced increase in the plasma concentration and urinary excretion of purine bases. Metabolism. 1996 Nov;45(11):1354-9. [8931639 ]
- Schutgens RB, Wanders RJ, Heymans HS, Schram AW, Tager JM, Schrakamp G, van den Bosch H: Zellweger syndrome: biochemical procedures in diagnosis, prevention and treatment. J Inherit Metab Dis. 1987;10 Suppl 1:33-45. [3119940 ]
|
|---|
| Synthesis Reference: |
Ballou, Clinton E.; Fischer, Hermann O. L. The synthesis of dihydroxyacetone phosphate. Journal of the American Chemical Society (1956), 78 1659-61. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|