|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110135 |
|---|
|
Identification |
|---|
| Name: |
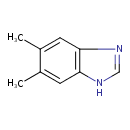
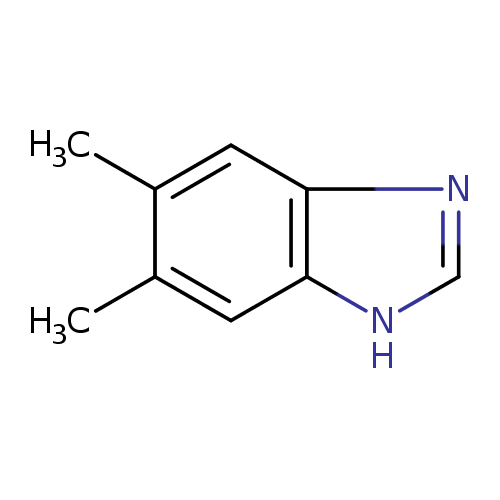
5,6-dimethylbenzimidazole |
|---|
| Description: | A dimethylbenzimidazole carrying methyl substituents at positions 5 and 6. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
DMB
-
dimethylbenzimidazole
|
|---|
|
Chemical Formula: |
C9H10N2
|
|---|
| Average Molecular Weight: |
146.19 |
|---|
| Monoisotopic Molecular
Weight: |
146.0843983314 |
|---|
| InChI Key: |
LJUQGASMPRMWIW-UHFFFAOYSA-N |
|---|
| InChI: |
InChI=1S/C9H10N2/c1-6-3-8-9(4-7(6)2)11-5-10-8/h3-5H,1-2H3,(H,10,11) |
|---|
| CAS
number: |
582-60-5 |
|---|
| IUPAC Name: | 5,6-dimethyl-1H-benzimidazole |
|---|
|
Traditional IUPAC Name: |
5,6-dimethylbenzimidazole |
|---|
| SMILES: | CC2(C(C)=CC1(=C(N=CN1)C=2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as benzimidazoles. These are organic compounds containing a benzene ring fused to an imidazole ring (five member ring containing a nitrogen atom, 4 carbon atoms, and two double bonds). |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Benzimidazoles |
|---|
|
Direct Parent |
Benzimidazoles |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Benzimidazole
- Benzenoid
- Heteroaromatic compound
- Imidazole
- Azole
- Azacycle
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
205.5 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 205.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 1.854 | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Schipp CJ, Marco-Urrea E, Kublik A, Seifert J, Adrian L (2013)Organic cofactors in the metabolism of Dehalococcoides mccartyi strains. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 368, Pubmed: 23479751
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|