|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110128 |
|---|
|
Identification |
|---|
| Name: |
porphobilinogen |
|---|
| Description: | Conjugate base of porphobilinogen arising from deprotonation of the two carboxy groups and protonation of the amino group; major species at pH 7.3. |
|---|
|
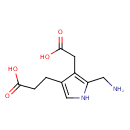
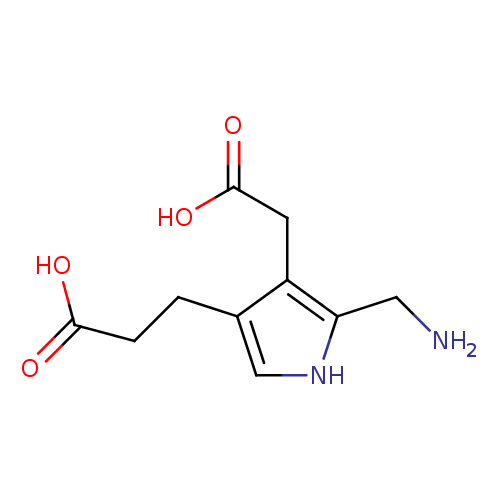
Structure |
|
|---|
| Synonyms: | - 5-(Aminomethyl)-4-(carboxymethyl)-pyrrole-3-propionate
- 5-(Aminomethyl)-4-(carboxymethyl)-pyrrole-3-propionic acid
- PBG
|
|---|
|
Chemical Formula: |
C10H13N2O4
|
|---|
| Average Molecular Weight: |
225.22 |
|---|
| Monoisotopic Molecular
Weight: |
227.1031819803 |
|---|
| InChI Key: |
QSHWIQZFGQKFMA-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/C10H14N2O4/c11-4-8-7(3-10(15)16)6(5-12-8)1-2-9(13)14/h5,12H,1-4,11H2,(H,13,14)(H,15,16)/p-1 |
|---|
| CAS
number: |
487-90-1 |
|---|
| IUPAC Name: | 3-[5-(azaniumylmethyl)-4-(carboxylatomethyl)-1H-pyrrol-3-yl]propanoate |
|---|
|
Traditional IUPAC Name: |
porphobilinogen |
|---|
| SMILES: | C(C1(=C(C(=CN1)CCC(=O)[O-])CC(=O)[O-]))[N+] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as aralkylamines. These are alkylamines in which the alkyl group is substituted at one carbon atom by an aromatic hydrocarbyl group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic nitrogen compounds |
|---|
| Sub Class | Organonitrogen compounds |
|---|
|
Direct Parent |
Aralkylamines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Aralkylamine
- Dicarboxylic acid or derivatives
- Substituted pyrrole
- Heteroaromatic compound
- Pyrrole
- Amino acid
- Amino acid or derivatives
- Carboxylic acid derivative
- Azacycle
- Carboxylic acid
- Organoheterocyclic compound
- Organopnictogen compound
- Primary amine
- Organooxygen compound
- Primary aliphatic amine
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Dhar GJ, Bossenmaier I, Petryka ZJ, Cardinal R, Watson CJ: Effects of hematin in hepatic porphyria. Further studies. Ann Intern Med. 1975 Jul;83(1):20-30. [1147435 ]
- Ivanov E, Pisanets M: Studies on the biosynthesis of porphyrins in erythrocytes after incubation with delta-aminolevulinic acid: an attempt to investigate the pathogenesis of nephrogenic anemia. Acta Biol Med Ger. 1982;41(4):307-13. [7124248 ]
- Ellencweig N, Schoenfeld N, Zemishlany Z: Acute intermittent porphyria: psychosis as the only clinical manifestation. Isr J Psychiatry Relat Sci. 2006;43(1):52-6. [16910386 ]
- Buchet JP, Lauwerys R, Hassoun A, Dratwa M, Wens R, Collart F, Tielemans C: Effect of aluminum on porphyrin metabolism in hemodialyzed patients. Nephron. 1987;46(4):360-3. [3658064 ]
- Tishler PV, Woodward B, O'Connor J, Holbrook DA, Seidman LJ, Hallett M, Knighton DJ: High prevalence of intermittent acute porphyria in a psychiatric patient population. Am J Psychiatry. 1985 Dec;142(12):1430-6. [4073306 ]
- Hsiao KJ, Lee FY, Wu SJ, Chang WJ: Determination of erythrocyte porphobilinogen deaminase activity using porphobilinogen as substrate. Clin Chim Acta. 1987 Sep 30;168(2):257-8. [3677422 ]
- Evans J, Lefkowitch J, Lim CK, Billing B: Fecal porphyrin abnormalities in a patient with features of Rotor's syndrome. Gastroenterology. 1981 Dec;81(6):1125-30. [7286590 ]
- Sassa S, Solish G, Levere RD, Kappas A: Studies in porphyria. IV. Expression of the gene defect of acute intermittent porphyria in cultured human skin fibroblasts and amniotic cells: prenatal diagnosis of the porphyric trait. J Exp Med. 1975 Sep 1;142(3):722-31. [1165472 ]
- Ford RE, Ou CN, Ellefson RD: Assay for erythrocyte uroporphyrinogen I synthase activity, with porphobilinogen as substrate. Clin Chem. 1980 Jul;26(8):1182-5. [7389090 ]
- Shiue JW, Lee FY, Hsiao KJ, Tsai YT, Lee SD, Wu SJ: Abnormal thyroid function and hypercholesterolemia in a case of acute intermittent porphyria. Taiwan Yi Xue Hui Za Zhi. 1989 Jul;88(7):729-31. [2809566 ]
- Mustajoki P: Normal erythrocyte uroporphyrinogen I synthase in a kindred with acute intermittent porphyria. Ann Intern Med. 1981 Aug;95(2):162-6. [7258864 ]
|
|---|
| Synthesis Reference: |
Frydman, Benjamin; Despuy, Maria E.; Rapoport, Henry. Pyrroles from azaindoles. A synthesis of porphobilinogen. Journal of the American Chemical Society (1965), 87(15), 3530-1. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|