|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110110 |
|---|
|
Identification |
|---|
| Name: |
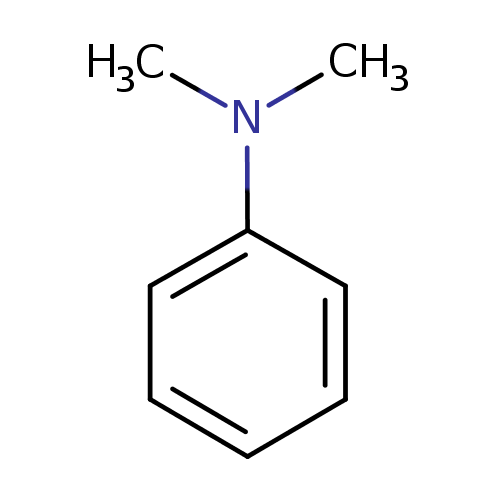
N,N-dimethylaniline |
|---|
| Description: | A tertiary amine that is aniline in which the amino hydrogens are replaced by two methyl groups. |
|---|
|
Structure |
|
|---|
| Synonyms: | - Dimethylaminobenzene
- Dimethylaniline
- Dimethylphenylamine
- N,N-Dimethyl-N-phenylamine
- N,N-Dimethylbenzenamine
- N,N-Dimethylbenzeneamine
- N,N-Dimethylphenylamine
- N,N-(dimethylamino)Benzene
- N,N-Dimethyl-benzenamine
- N,N-Dimethylaniline sulfate (1:1)
- N,N-Dimethylaniline hydrochloride
- N,N-Dimethylaniline hydroiodide
|
|---|
|
Chemical Formula: |
C8H11N
|
|---|
| Average Molecular Weight: |
121.18 |
|---|
| Monoisotopic Molecular
Weight: |
121.0891493583 |
|---|
| InChI Key: |
JLTDJTHDQAWBAV-UHFFFAOYSA-N |
|---|
| InChI: |
InChI=1S/C8H11N/c1-9(2)8-6-4-3-5-7-8/h3-7H,1-2H3 |
|---|
| CAS
number: |
121-69-7 |
|---|
| IUPAC Name: | N,N-dimethylaniline |
|---|
|
Traditional IUPAC Name: |
dimethylaniline |
|---|
| SMILES: | CN(C1(C=CC=CC=1))C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as dialkylarylamines. These are aliphatic aromatic amines in which the amino group is linked to two aliphatic chains and one aromatic group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic nitrogen compounds |
|---|
| Sub Class | Organonitrogen compounds |
|---|
|
Direct Parent |
Dialkylarylamines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Aniline or substituted anilines
- Dialkylarylamine
- Benzenoid
- Monocyclic benzene moiety
- Organopnictogen compound
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
2.5 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 2.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 1.45 mg/mL | Not Available | | LogP | 2.31 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-00di-0900000000-5b63cd88da62acd2d77f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0a4i-0900000000-31c3fe91c89abc5104e6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-004i-9200000000-f36bb1867752d2b67e26 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-6L) , Positive | splash10-00di-1900000000-544ffdcec51667ffcedc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80) , Positive | splash10-00di-5900000000-60173199ae4aeb84fe4e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-7M) , Positive | splash10-00di-4900000000-7a8c56c9a1e904571576 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-00di-0900000000-3cec8f9949b4ca1fda59 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-0a4i-0900000000-1402f1580a4bb8ce5135 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-0a4i-0900000000-e2755a3a974bacd7cdf1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-0a4i-1900000000-0c939551fc3df2940361 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-056r-9500000000-d7dca8a17ef3b18f33fd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-b3487e6b57de233c5872 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0900000000-417b2289cb697ca4ac57 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zmi-9500000000-a5d45941d30346a15ebc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-cc4c1f59bd103642f74c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-1900000000-9eceba6cab47009c7828 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0umi-6900000000-b85e09230bdd448d6ee5 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Sponza DT, Oztekin R (2014)Dephenolization, dearomatization and detoxification of olive mill wastewater with sonication combined with additives and radical scavengers. Ultrasonics sonochemistry 21, Pubmed: 24315030
|
|---|
| Synthesis Reference: |
Li, Guo-tao; Guan, Nai-jia; Zhang, Huai-bin; Liu, Shu-quan. Synthesis of N, N-dimethylaniline by aniline and methanol over b zeolite catalyst. Shiyou Huagong (2002), 31(2), 81-83. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|