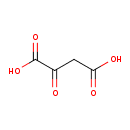
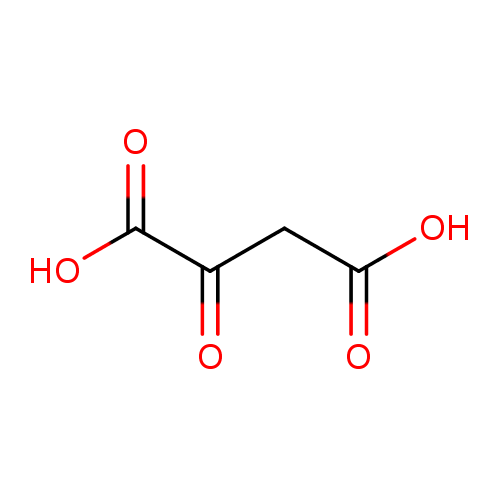
| References: |
- Wolfenden R, Lewis CA, Yuan Y (2011)Kinetic challenges facing oxalate, malonate, acetoacetate, and oxaloacetate decarboxylases. Journal of the American Chemical Society 133, Pubmed: 21434608
- Kinoshita H, Nagasaki J, Yoshikawa N, Yamamoto A, Takito S, Kawasaki M, Sugiyama T, Miyake H, Weber AP, Taniguchi M (2011)The chloroplastic 2-oxoglutarate/malate transporter has dual function as the malate valve and in carbon/nitrogen metabolism. The Plant journal : for cell and molecular biology 65, Pubmed: 21175886
- Tomasiak TM, Archuleta TL, Andréll J, Luna-Chávez C, Davis TA, Sarwar M, Ham AJ, McDonald WH, Yankovskaya V, Stern HA, Johnston JN, Maklashina E, Cecchini G, Iverson TM (2011)Geometric restraint drives on- and off-pathway catalysis by the Escherichia coli menaquinol:fumarate reductase. The Journal of biological chemistry 286, Pubmed: 21098488
- Williams DS, Cash A, Hamadani L, Diemer T (2009)Oxaloacetate supplementation increases lifespan in Caenorhabditis elegans through an AMPK/FOXO-dependent pathway. Aging cell 8, Pubmed: 19793063
- Luque-Almagro VM, Merchán F, Blasco R, Igeńo MI, Martínez-Luque M, Moreno-Vivián C, Castillo F, Roldán MD (2011)Cyanide degradation by Pseudomonas pseudoalcaligenes CECT5344 involves a malate:quinone oxidoreductase and an associated cyanide-insensitive electron transfer chain. Microbiology (Reading, England) 157, Pubmed: 21178163
- Carvalho AS, Torres LB, Persike DS, Fernandes MJ, Amado D, Naffah-Mazzacoratti Mda G, Cavalheiro EA, da Silva AV (2011)Neuroprotective effect of pyruvate and oxaloacetate during pilocarpine induced status epilepticus in rats. Neurochemistry international 58, Pubmed: 21185899
- Wang Y, Blatt MR (2011)Anion channel sensitivity to cytosolic organic acids implicates a central role for oxaloacetate in integrating ion flux with metabolism in stomatal guard cells. The Biochemical journal 439, Pubmed: 21745184
- Stipanovic RD, Wheeler MH, Puckhaber LS, Liu J, Bell AA, Williams HJ (2011)Nuclear magnetic resonance (NMR) studies on the biosynthesis of fusaric acid from Fusarium oxysporum f. sp. vasinfectum. Journal of agricultural and food chemistry 59, Pubmed: 21495723
|
|---|