|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110101 |
|---|
|
Identification |
|---|
| Name: |
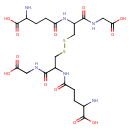
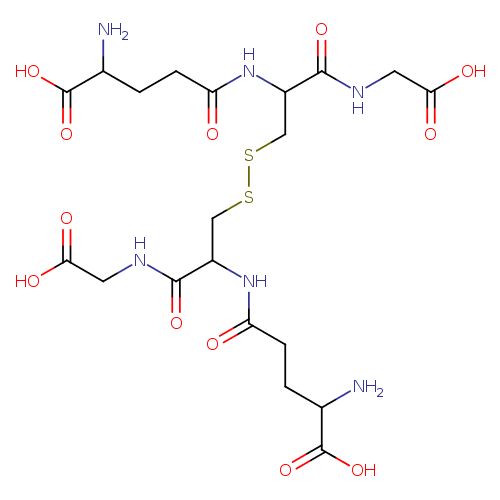
glutathione disulfide |
|---|
| Description: | A doubly-charged peptide anion arising from deprotonation of the four carboxy groups and protonation of the two amino groups of glutathione disulfide; major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
glutathione oxidized
-
glutathione ox
-
GSSG
-
oxidized glutathione
|
|---|
|
Chemical Formula: |
C20H30N6O12S2
|
|---|
| Average Molecular Weight: |
610.61 |
|---|
| Monoisotopic Molecular
Weight: |
614.1676119678 |
|---|
| InChI Key: |
YPZRWBKMTBYPTK-BJDJZHNGSA-L |
|---|
| InChI: |
InChI=1S/C20H32N6O12S2/c21-9(19(35)36)1-3-13(27)25-11(17(33)23-5-15(29)30)7-39-40-8-12(18(34)24-6-16(31)32)26-14(28)4-2-10(22)20(37)38/h9-12H,1-8,21-22H2,(H,23,33)(H,24,34)(H,25,27)(H,26,28)(H,29,30)(H,31,32)(H,35,36)(H,37,38)/p-2/t9-,10-,11-,12-/m0/s1 |
|---|
| CAS
number: |
27025-41-8 |
|---|
| IUPAC Name: | (2S,2'S)- 5,5'- 5,5'- [disulfanediylbis({(2R)- [disulfanediylbis({(2R)- 3- 3- [(carboxylatomethyl)amino]- [(carboxylatomethyl)amino]- 3- 3- oxopropane- oxopropane- 1,2- 1,2- diyl}imino)]bis(2- diyl}imino)]bis(2- azaniumyl- azaniumyl- 5- 5- oxopentanoate) oxopentanoate) |
|---|
|
Traditional IUPAC Name: |
oxiglutatione |
|---|
| SMILES: | C(SSCC(C(NCC([O-])=O)=O)NC(=O)CCC([N+])C([O-])=O)C(C(NCC([O-])=O)=O)NC(=O)CCC([N+])C([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Oligopeptides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-oligopeptide
- Gamma-glutamyl alpha peptide
- Glutamine or derivatives
- N-acyl-alpha-amino acid
- Tetracarboxylic acid or derivatives
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- Cysteine or derivatives
- Alpha-amino acid
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- Fatty amide
- N-acyl-amine
- Fatty acyl
- Amino acid or derivatives
- Carboxamide group
- Amino acid
- Dialkyldisulfide
- Secondary carboxylic acid amide
- Organic disulfide
- Sulfenyl compound
- Carboxylic acid
- Organopnictogen compound
- Primary aliphatic amine
- Hydrocarbon derivative
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Organic oxide
- Amine
- Organonitrogen compound
- Organooxygen compound
- Organosulfur compound
- Primary amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kondo T, Ohtsuka Y, Shimada M, Kawakami Y, Hiyoshi Y, Tsuji Y, Fujii H, Miwa S: Erythrocyte-oxidized glutathione transport in pyrimidine 5'-nucleotidase deficiency. Am J Hematol. 1987 Sep;26(1):37-45. [2888306 ]
- Calabrese V, Scapagnini G, Ravagna A, Bella R, Butterfield DA, Calvani M, Pennisi G, Giuffrida Stella AM: Disruption of thiol homeostasis and nitrosative stress in the cerebrospinal fluid of patients with active multiple sclerosis: evidence for a protective role of acetylcarnitine. Neurochem Res. 2003 Sep;28(9):1321-8. [12938853 ]
- Tauler P, Sureda A, Cases N, Aguilo A, Rodriguez-Marroyo JA, Villa G, Tur JA, Pons A: Increased lymphocyte antioxidant defences in response to exhaustive exercise do not prevent oxidative damage. J Nutr Biochem. 2006 Oct;17(10):665-71. Epub 2005 Nov 28. [16481153 ]
- Slivka A, Spina MB, Cohen G: Reduced and oxidized glutathione in human and monkey brain. Neurosci Lett. 1987 Feb 10;74(1):112-8. [3561870 ]
- Aukrust P, Svardal AM, Muller F, Lunden B, Berge RK, Ueland PM, Froland SS: Increased levels of oxidized glutathione in CD4+ lymphocytes associated with disturbed intracellular redox balance in human immunodeficiency virus type 1 infection. Blood. 1995 Jul 1;86(1):258-67. [7795231 ]
- Sakhi AK, Russnes KM, Smeland S, Blomhoff R, Gundersen TE: Simultaneous quantification of reduced and oxidized glutathione in plasma using a two-dimensional chromatographic system with parallel porous graphitized carbon columns coupled with fluorescence and coulometric electrochemical detection. J Chromatogr A. 2006 Feb 3;1104(1-2):179-89. [16376913 ]
- Glazyrin AL, Kolesnikov SI, Safronov AYu: Histochemical localization of oxidized glutathione-catalysing enzymes in human term placenta. Histochem J. 1993 Jan;25(1):45-50. [8432663 ]
- Carru C, Zinellu A, Pes GM, Marongiu G, Tadolini B, Deiana L: Ultrarapid capillary electrophoresis method for the determination of reduced and oxidized glutathione in red blood cells. Electrophoresis. 2002 Jun;23(11):1716-21. [12179993 ]
- Tohgi H, Abe T, Saheki M, Hamato F, Sasaki K, Takahashi S: Reduced and oxidized forms of glutathione and alpha-tocopherol in the cerebrospinal fluid of parkinsonian patients: comparison between before and after L-dopa treatment. Neurosci Lett. 1995 Jan 16;184(1):21-4. [7739798 ]
- Yoshida T: Determination of reduced and oxidized glutathione in erythrocytes by high-performance liquid chromatography with ultraviolet absorbance detection. J Chromatogr B Biomed Appl. 1996 Apr 12;678(2):157-64. [8738017 ]
- Sofic E, Lange KW, Jellinger K, Riederer P: Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson's disease. Neurosci Lett. 1992 Aug 17;142(2):128-30. [1454205 ]
- Muda P, Kampus P, Zilmer M, Zilmer K, Kairane C, Ristimae T, Fischer K, Teesalu R: Homocysteine and red blood cell glutathione as indices for middle-aged untreated essential hypertension patients. J Hypertens. 2003 Dec;21(12):2329-33. [14654754 ]
- Satoh T, Yoshioka Y: Contribution of reduced and oxidized glutathione to signals detected by magnetic resonance spectroscopy as indicators of local brain redox state. Neurosci Res. 2006 May;55(1):34-9. Epub 2006 Feb 24. [16503064 ]
- Srivastava SK, Beutler E: Oxidized glutathione levels in erythrocytes of glucose-6-phosphate-dehydrogenase-deficient subjects. Lancet. 1968 Jul 6;2(7558):23-4. [4172687 ]
- Board P, Nishida T, Gatmaitan Z, Che M, Arias IM: Erythrocyte membrane transport of glutathione conjugates and oxidized glutathione in the Dubin-Johnson syndrome and in rats with hereditary hyperbilirubinemia. Hepatology. 1992 Apr;15(4):722-5. [1551648 ]
|
|---|
| Synthesis Reference: |
Saito, Susumu; Nishijima, Kunihide; Kataoka, Katsuyuki; Aoyanagi, Yoshinori; Fukuda, Yoji; Ito, Homare. Manufacture of oxidized glutathione from reduced glutathione with ascorbic acid and ascorbate oxidase. Jpn. Kokai Tokkyo Koho (1995), 4 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|