|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110100 |
|---|
|
Identification |
|---|
| Name: |
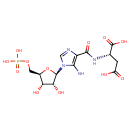
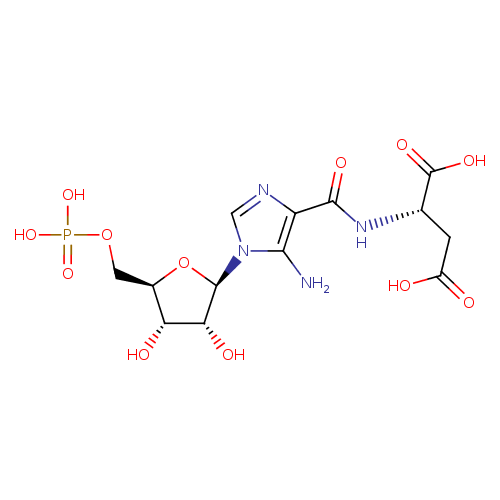
5'-phosphoribosyl-4-(N-succinocarboxamide)-5-aminoimidazole |
|---|
| Description: | An organophosphate oxoanion that is the tetraanionic form of SAICAR. It is the major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
(S)-2-[5-amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamido]succinate
-
1-(5-phosphoribosyl)-4-(N-succino-carboxamide)-5-aminoimidazole
-
5'-P-ribosyl-4-(N-succinocarboxamide)-5-aminoimidazole
-
5'-p-Ribosyl-4-(N-succinocarboxamide)-5-amino imidazole
-
1-(5'-phosphoribosyl)-4-(N-succino-carboxamide)-5-aminoimidazole
-
SAICAR
|
|---|
|
Chemical Formula: |
C13H15N4O12P
|
|---|
| Average Molecular Weight: |
450.26 |
|---|
| Monoisotopic Molecular
Weight: |
454.0737086059 |
|---|
| InChI Key: |
NAQGHJTUZRHGAC-LBGUGVGYSA-J |
|---|
| InChI: |
InChI=1S/C13H19N4O12P/c14-10-7(11(22)16-4(13(23)24)1-6(18)19)15-3-17(10)12-9(21)8(20)5(29-12)2-28-30(25,26)27/h3-5,8-9,12,20-21H,1-2,14H2,(H,16,22)(H,18,19)(H,23,24)(H2,25,26,27)/p-4/t4?,5-,8-,9-,12-/m1/s1 |
|---|
| CAS
number: |
3031-95-6 |
|---|
| IUPAC Name: | (2S)- 2- 2- [5- [5- amino- amino- 1- 1- (5- (5- O- O- phosphonato- phosphonato- β- β- D- D- ribosyl)imidazole- ribosyl)imidazole- 4- 4- carboxamido]succinate carboxamido]succinate |
|---|
|
Traditional IUPAC Name: |
saicar |
|---|
| SMILES: | C(OP([O-])([O-])=O)C2(C(O)C(O)C(N1(C(N)=C(C(=O)NC(C([O-])=O)CC([O-])=O)N=C1))O2) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as 1-ribosyl-imidazolecarboxamides. These are organic compounds containing the imidazole ring linked to a ribose ring through a 1-2 bond. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Imidazole ribonucleosides and ribonucleotides |
|---|
|
Direct Parent |
1-ribosyl-imidazolecarboxamides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 1-ribosyl-imidazolecarboxamide
- Pentose phosphate
- Pentose-5-phosphate
- Aspartic acid or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- Alpha-amino acid or derivatives
- Monosaccharide phosphate
- Pentose monosaccharide
- 2-heteroaryl carboxamide
- Imidazolyl carboxylic acid derivative
- Imidazole-4-carbonyl group
- Monoalkyl phosphate
- Alkyl phosphate
- Organic phosphoric acid derivative
- Primary aromatic amine
- Dicarboxylic acid or derivatives
- Phosphoric acid ester
- N-substituted imidazole
- Aminoimidazole
- Monosaccharide
- Imidazole
- Heteroaromatic compound
- Azole
- Vinylogous amide
- Oxolane
- Amino acid or derivatives
- Carboxamide group
- Amino acid
- Secondary carboxylic acid amide
- Secondary alcohol
- 1,2-diol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Carboxylic acid
- Alcohol
- Organic oxygen compound
- Carbonyl group
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Amine
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Keller KE, Tan IS, Lee YS (2012)SAICAR stimulates pyruvate kinase isoform M2 and promotes cancer cell survival in glucose-limited conditions. Science (New York, N.Y.) 338, Pubmed: 23086999
- Keller KE, Doctor ZM, Dwyer ZW, Lee YS (2014)SAICAR induces protein kinase activity of PKM2 that is necessary for sustained proliferative signaling of cancer cells. Molecular cell 53, Pubmed: 24606918
|
|---|
| Synthesis Reference: |
Shaw, Gordon; Thomas, Peter S.; Patey, Carole A. H.; Thomas, Susan E. Purines, pyrimidines and imidazoles. Part 50. Inhibition of adenylosuccinate AMP-lyase no. 4.3.2.2. by derivatives of N-(5-amino-1-b-D-ribofuranosylimidazole-4-carbonyl)-L-aspartic acid 5'-phosphate (SAICAR) and virazole 5'-phosphate. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999) (1979), (6), 1415-24. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|