|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110083 |
|---|
|
Identification |
|---|
| Name: |
carbamoyl-phosphate |
|---|
| Description: | A doubly-charged organophosphate oxoanion arising from deprotonation of the phosphate OH groups of carbamoyl phosphate; major species at pH 7.3. |
|---|
|
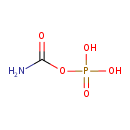
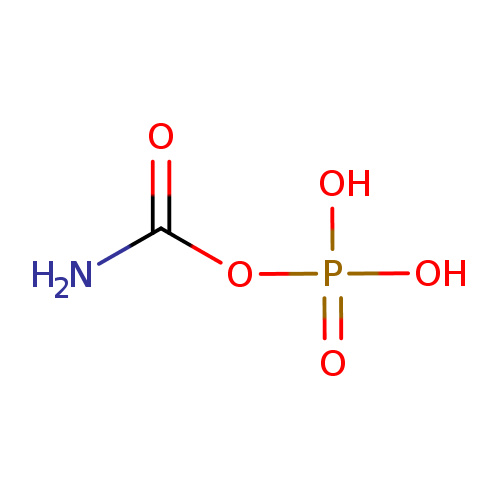
Structure |
|
|---|
| Synonyms: | -
carbamoyl-P
-
carbamyl-phosphate
|
|---|
|
Chemical Formula: |
CH2NO5P
|
|---|
| Average Molecular Weight: |
139 |
|---|
| Monoisotopic Molecular
Weight: |
140.9827087541 |
|---|
| InChI Key: |
FFQKYPRQEYGKAF-UHFFFAOYSA-L |
|---|
| InChI: |
InChI=1S/CH4NO5P/c2-1(3)7-8(4,5)6/h(H2,2,3)(H2,4,5,6)/p-2 |
|---|
| CAS
number: |
590-55-6 |
|---|
| IUPAC Name: | carbamoyl phosphate |
|---|
|
Traditional IUPAC Name: |
carbamoyl-phosphate |
|---|
| SMILES: | C(=O)(N)OP(=O)([O-])[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as organic phosphoric acids and derivatives. These are organic compounds containing phosphoric acid or a derivative thereof. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Organic phosphoric acids and derivatives |
|---|
|
Direct Parent |
Organic phosphoric acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Organic phosphoric acid derivative
- Carboximidic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Imine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Sigoillot FD, Kotsis DH, Serre V, Sigoillot SM, Evans DR, Guy HI: Nuclear localization and mitogen-activated protein kinase phosphorylation of the multifunctional protein CAD. J Biol Chem. 2005 Jul 8;280(27):25611-20. Epub 2005 May 12. [15890648 ]
- Struck J, Uhlein M, Morgenthaler NG, Furst W, Hoflich C, Bahrami S, Bergmann A, Volk HD, Redl H: Release of the mitochondrial enzyme carbamoyl phosphate synthase under septic conditions. Shock. 2005 Jun;23(6):533-8. [15897806 ]
- Schnater JM, Bruder E, Bertschin S, Woodtli T, de Theije C, Pietsch T, Aronson DC, von Schweinitz D, Lamers WH, Kohler ES: Subcutaneous and intrahepatic growth of human hepatoblastoma in immunodeficient mice. J Hepatol. 2006 Sep;45(3):377-86. Epub 2006 May 3. [16780998 ]
- Chen KF, Lai YY, Sun HS, Tsai SJ: Transcriptional repression of human cad gene by hypoxia inducible factor-1alpha. Nucleic Acids Res. 2005 Sep 9;33(16):5190-8. Print 2005. [16155188 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|