|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110078 |
|---|
|
Identification |
|---|
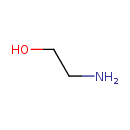
| Name: |
ethanolamine |
|---|
| Description: | A primary aliphatic ammonium ion that is the conjugate acid of ethanolamine arising from protonation of the primary amino function. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
2-aminoethanol
-
monoethanolamine
-
colamine
-
ethanol-amine
|
|---|
|
Chemical Formula: |
C2H8NO
|
|---|
| Average Molecular Weight: |
62.091 |
|---|
| Monoisotopic Molecular
Weight: |
62.0605888841 |
|---|
| InChI Key: |
HZAXFHJVJLSVMW-UHFFFAOYSA-O |
|---|
| InChI: |
InChI=1S/C2H7NO/c3-1-2-4/h4H,1-3H2/p+1 |
|---|
| CAS
number: |
141-43-5 |
|---|
| IUPAC Name: | 2-hydroxyethanaminium |
|---|
|
Traditional IUPAC Name: |
ethanolamine |
|---|
| SMILES: | C(CO)[N+] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as 1,2-aminoalcohols. These are organic compounds containing an alkyl chain with an amine group bound to the C1 atom and an alcohol group bound to the C2 atom. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
|
Class |
Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
|
Direct Parent |
1,2-aminoalcohols |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 1,2-aminoalcohol
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary amine
- Primary alcohol
- Organooxygen compound
- Primary aliphatic amine
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | +1 |
|---|
|
Melting point: |
10.5 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 10.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 1000.0 mg/mL | Not Available | | LogP | -1.31 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-1900000000-b64e859a0bfc46cdfcbf | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-1900000000-384c99d021f0303a9d78 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-00di-1900000000-d731dd07c2dfa0287f5f | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-8900000000-ec4268b6041043d15437 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-00di-2900000000-02697b8ce238020537aa | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-03dl-9000000000-17dee0c07bf5cddb79ce | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-03di-9000000000-04f4c1792f8ab4f4646f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-03di-9000000000-761836be8f1018081210 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-7M) , Positive | splash10-001i-9000000000-7160c3fea0c0159e447a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-68) , Positive | splash10-001i-9000000000-2af16a98e43fadfa86a3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-03di-9000000000-ae5b80bd3c5c11914ea6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-0006-9000000000-6abb3d0944e188778c65 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-0007-9000000000-e477e52f411d933a30b7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-0006-9000000000-d7979261a3716365d5ae | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-03di-9000000000-5c0261b2dcc6ae1291a1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-9000000000-9e6d219a7fbd8624cd6b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ox-9000000000-fb9020ee9b301ec7766f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-a4ae359cfcc0245b4d19 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-9000000000-b6ed81bc049b8d3042f6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dl-9000000000-78c77096ed599182b5bb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-f199cbf8312e0b466f1b | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-001i-9000000000-b8e7ed9f5ad724511431 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Engelborghs S, Marescau B, De Deyn PP: Amino acids and biogenic amines in cerebrospinal fluid of patients with Parkinson's disease. Neurochem Res. 2003 Aug;28(8):1145-50. [12834252 ]
- Denissen JF, Grabowski BA, Johnson MK, Boyd SA, Uchic JT, Stein H, Cepa S, Hill P: The orally active renin inhibitor A-74273. In vivo and in vitro morpholine ring metabolism in rats, dogs, and humans. Drug Metab Dispos. 1994 Nov-Dec;22(6):880-8. [7895605 ]
- Vance JE: Lipoproteins secreted by cultured rat hepatocytes contain the antioxidant 1-alk-1-enyl-2-acylglycerophosphoethanolamine. Biochim Biophys Acta. 1990 Jul 16;1045(2):128-34. [2116174 ]
- Alberghina M, Giacchetto A, Cavallaro N: Levels of ethanolamine intermediates in the human and rat visual system structures: comparison with neural tissues of a lower vertebrate (Mustelus canis) and an invertebrate (Loligo pealei). Neurochem Int. 1993 Jan;22(1):45-51. [8443564 ]
- Hammond EJ, Uthman BM, Wilder BJ, Ben-Menachem E, Hamberger A, Hedner T, Ekman R: Neurochemical effects of vagus nerve stimulation in humans. Brain Res. 1992 Jun 26;583(1-2):300-3. [1504837 ]
- Perschak H, Amsler U, Vischer A, Siegfried J, Cuenod M: Ventricular cerebrospinal fluid concentrations of putative amino acid transmitters in Parkinson's disease and other disorders. Hum Neurobiol. 1987;6(3):191-4. [2896652 ]
- Renkonen O: Chromatographic separation of plasmalogenic, alkyl-acyl, and diacyl forms of ethanolamine glycerophosphatides. J Lipid Res. 1968 Jan;9(1):34-9. [4295349 ]
- Ginsberg L, Rafique S, Xuereb JH, Rapoport SI, Gershfeld NL: Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer's disease brain. Brain Res. 1995 Nov 6;698(1-2):223-6. [8581486 ]
- Bluml S, Seymour KJ, Ross BD: Developmental changes in choline- and ethanolamine-containing compounds measured with proton-decoupled (31)P MRS in in vivo human brain. Magn Reson Med. 1999 Oct;42(4):643-54. [10502752 ]
- Farooqui AA, Rapoport SI, Horrocks LA: Membrane phospholipid alterations in Alzheimer's disease: deficiency of ethanolamine plasmalogens. Neurochem Res. 1997 Apr;22(4):523-7. [9130265 ]
- Mikhaevich IS, Vlasenkova NK, Gerasimova GK: Synergistic antiproliferative effect of cis-diammine-dichloroplatinum (II) and a new anticancer agent, plasmanyl-(N-acyl)-ethanolamine, an inhibitor of protein kinase C. Biomed Sci. 1991;2(6):659-64. [1841636 ]
|
|---|
| Synthesis Reference: |
Soucaille, Philippe. Ethanolamine production by fermentation of genetically modified Escherichia coli. PCT Int. Appl. (2007), 23pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|