|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110077 |
|---|
|
Identification |
|---|
| Name: |
(3R,5S)-1-pyrroline-3-hydroxy-5-carboxylate |
|---|
| Description: | The 1-pyrrolinecarboxylate formed by deprotonation of the carboxy group of (3R,5S)-1-pyrroline-3-hydroxy-5-carboxylic acid; principal microspecies at pH 7.3. |
|---|
|
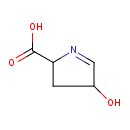
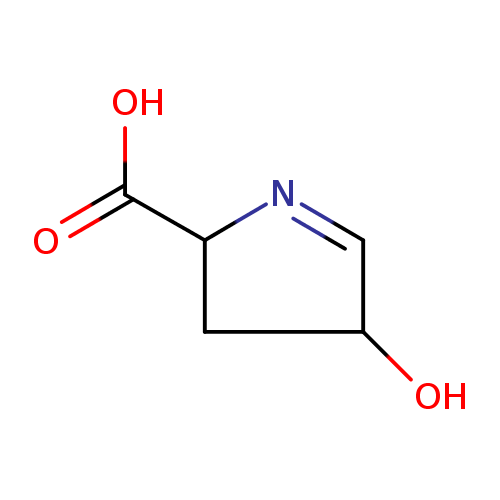
Structure |
|
|---|
| Synonyms: | -
L-Δ1-pyrroline 3-hydroxy-5-carboxylate
-
L-1pyrroline-3-hydroxy-5-carboxylate
|
|---|
|
Chemical Formula: |
C5H6NO3
|
|---|
| Average Molecular Weight: |
128.11 |
|---|
| Monoisotopic Molecular
Weight: |
129.0425930962 |
|---|
| InChI Key: |
WFOFKRKDDKGRIK-DMTCNVIQSA-M |
|---|
| InChI: |
InChI=1S/C5H7NO3/c7-3-1-4(5(8)9)6-2-3/h2-4,7H,1H2,(H,8,9)/p-1/t3-,4+/m1/s1 |
|---|
| CAS
number: |
22573-88-2 |
|---|
| IUPAC Name: | (2S,4R)-4-hydroxy-3,4-dihydro-2H-pyrrole-2-carboxylate |
|---|
|
Traditional IUPAC Name: |
pyrroline-hydroxy-carboxylate |
|---|
| SMILES: | C1(=NC(C([O-])=O)CC(O)1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as alpha amino acids and derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon), or a derivative thereof. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Alpha amino acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-amino acid or derivatives
- Pyrroline carboxylic acid
- Pyrroline carboxylic acid or derivatives
- Pyrroline
- Secondary alcohol
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Organoheterocyclic compound
- Azacycle
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Organic nitrogen compound
- Carbonyl group
- Alcohol
- Imine
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Arginine and Proline Metabolism pae00330
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Simila S: Hydroxyproline metabolism in type II hyperprolinaemia. Ann Clin Biochem. 1979 Jul;16(4):177-81. [533224 ]
- Onenli-Mungan N, Yuksel B, Elkay M, Topaloglu AK, Baykal T, Ozer G: Type II hyperprolinemia: a case report. Turk J Pediatr. 2004 Apr-Jun;46(2):167-9. [15214748 ]
- Valle D, Goodman SI, Harris SC, Phang JM: Genetic evidence for a common enzyme catalyzing the second step in the degradation of proline and hydroxyproline. J Clin Invest. 1979 Nov;64(5):1365-70. [500817 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|