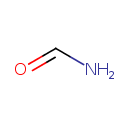
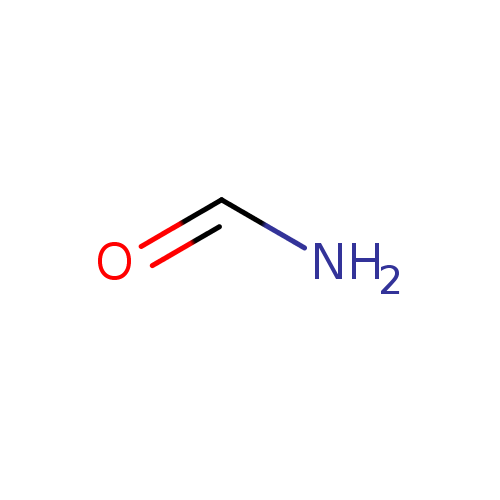
| References: |
- Augustine-Rauch KA, Zhang Q, Kleinman M, Lawton R, Welsh MJ (2004)A study of vehicles for dosing rodent whole embryo culture with non aqueous soluble compounds. Reproductive toxicology (Elmsford, N.Y.) 18, Pubmed: 15082074
- Li J (2002)Elimination of polymer interference in chromatographic analysis of estradiol degradation products in a transdermal drug delivery formulation by proper selection of extraction solvents. Journal of pharmaceutical sciences 91, Pubmed: 12115814
- Sakthivel T, Jaitely V, Patel NV, Florence AT (2001)Non-aqueous emulsions: hydrocarbon-formamide systems. International journal of pharmaceutics 214, Pubmed: 11282235
- Falciola L, Mussini PR, Mussini T, Pelle P (2004)Determination of primary and secondary standards and characterization of appropriate salt bridges for pH measurements in formamide. Analytical chemistry 76, Pubmed: 14750843
- Simard C, Lemieux R, Côté S (2001)Urea substitutes toxic formamide as destabilizing agent in nucleic acid hybridizations with RNA probes. Electrophoresis 22, Pubmed: 11545392
- Nguyen VS, Abbott HL, Dawley MM, Orlando TM, Leszczynski J, Nguyen MT (2011)Theoretical study of formamide decomposition pathways. The journal of physical chemistry. A 115, Pubmed: 21229996
- Paarmann A, Lima M, Chelli R, Volkov VV, Righini R, Miller RJ (2011)Excitonic effects in two-dimensional vibrational spectra of liquid formamide. Physical chemistry chemical physics : PCCP 13, Pubmed: 21573300
- Kumaran R, Ramamurthy P (2011)Photophysical studies on the interaction of formamide and alkyl substituted amides with photoinduced electron transfer (PET) based acridinedione dyes in water. Journal of fluorescence 21, Pubmed: 21769603
- Hamann T, Edtbauer A, da Silva FF, Denifl S, Scheier P, Swiderek P (2011)Dissociative electron attachment to gas-phase formamide. Physical chemistry chemical physics : PCCP 13, Pubmed: 21647492
- Weiss AK, Hofer TS, Randolf BR, Bhattacharjee A, Rode BM (2011)Hydrogen bond formation of formamide and N-methylformamide in aqueous solution studied by quantum mechanical charge field-molecular dynamics (QMCF-MD). Physical chemistry chemical physics : PCCP 13, Pubmed: 21647491
- Karunasekara T, Poole CF (2011)Models for liquid-liquid partition in the system formamide-organic solvent and their use for estimating descriptors for organic compounds. Talanta 83, Pubmed: 21215846
- Liu Y, Hill BC (2007)Formamide probes a role for water in the catalytic cycle of cytochrome c oxidase. Biochimica et biophysica acta 1767, Pubmed: 17184725
- Zoranic L, Mazighi R, Sokolic F, Perera A (2009)Concentration fluctuations and microheterogeneity in aqueous amide mixtures. The Journal of chemical physics 130, Pubmed: 19334838
- Ferus M, Kubelík P, Civiš S (2011)Laser spark formamide decomposition studied by FT-IR spectroscopy. The journal of physical chemistry. A 115, Pubmed: 21932847
|
|---|