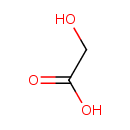
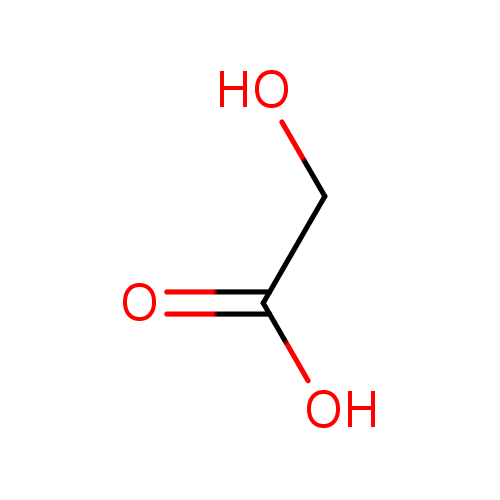
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-0002-0900000000-ed8b8e4a9e2556ea02e2 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-00dj-9600000000-8bafc88c7bf4e90fb5e8 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-003r-2910000000-bd50bf5bab6f5327eaf4 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-004i-9000000000-e942bdae1d60e5f5d649 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-00di-9000000000-f225de2de3540c3f50a4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-00di-9000000000-7de217d97b44f53aad82 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-00di-9000000000-88af2b259f82cd1d8938 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-004i-9000000000-c968a24f0640b154325b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0059-9000000000-1dfacf30bf94ce3bf8bb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-9000000000-d961c3c14ec415e3141e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-9000000000-67f73be970ba9f885c4a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-f2ccf0b88e0ad65ed4c6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-9000000000-7445713a5fe347bbc8b8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-26e13242443efc1aa846 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-6ba976b949118cd0a86a | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-001i-9000000000-2885890e3bb8c015742f | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
| References: |
- Salek RM, Maguire ML, Bentley E, Rubtsov DV, Hough T, Cheeseman M, Nunez D, Sweatman BC, Haselden JN, Cox RD, Connor SC, Griffin JL (2007)A metabolomic comparison of urinary changes in type 2 diabetes in mouse, rat, and human. Physiological genomics 29, Pubmed: 17190852
- Grostern A, Sales CM, Zhuang WQ, Erbilgin O, Alvarez-Cohen L (2012)Glyoxylate metabolism is a key feature of the metabolic degradation of 1,4-dioxane by Pseudonocardia dioxanivorans strain CB1190. Applied and environmental microbiology 78, Pubmed: 22327578
- Niessen M, Krause K, Horst I, Staebler N, Klaus S, Gaertner S, Kebeish R, Araujo WL, Fernie AR, Peterhansel C (2012)Two alanine aminotranferases link mitochondrial glycolate oxidation to the major photorespiratory pathway in Arabidopsis and rice. Journal of experimental botany 63, Pubmed: 22268146
- Schmitt DC, Malow EJ, Johnson JS (2012)Three-component glycolate Michael reactions of enolates, silyl glyoxylates, and a,ß-enones. The Journal of organic chemistry 77, Pubmed: 22394389
- Jiang J, Johnson LC, Knight J, Callahan MF, Riedel TJ, Holmes RP, Lowther WT (2012)Metabolism of [13C5]hydroxyproline in vitro and in vivo: implications for primary hyperoxaluria. American journal of physiology. Gastrointestinal and liver physiology 302, Pubmed: 22207577
- Knight J, Hinsdale M, Holmes R (2012)Glycolate and 2-phosphoglycolate content of tissues measured by ion chromatography coupled to mass spectrometry. Analytical biochemistry 421, Pubmed: 22093610
- Salido E, Pey AL, Rodriguez R, Lorenzo V (2012)Primary hyperoxalurias: disorders of glyoxylate detoxification. Biochimica et biophysica acta 1822, Pubmed: 22446032
|
|---|