|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100090 |
|---|
|
Identification |
|---|
| Name: |
Delta Biliverdin |
|---|
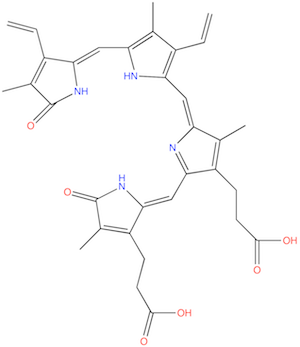
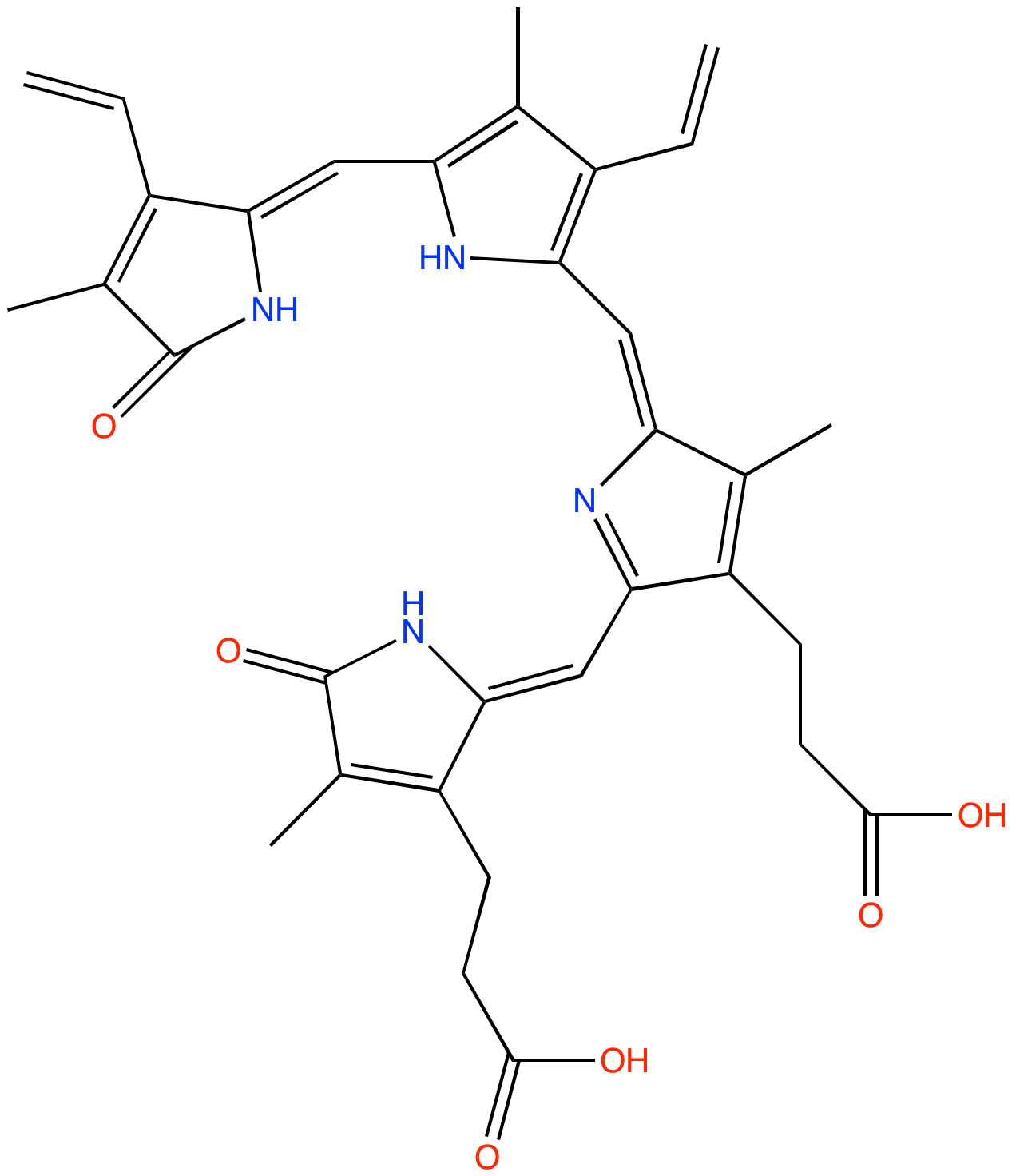
| Description: | Product of oxidative heme degradation produced by Pseudomonas aeruginosa. Produced via the catalytic activity of the Pseudomonas aeruginosa heme oxygenase (paHO) . Catalytic oxidative cleavage of heme to beta biliverdin by paHO facilitates the acquisition of iron by P. aeruginosa, and protects against redox-active "free heme." |
|---|
|
Structure |
|
|---|
| Synonyms: |
- Biliverdin IX delta
- 29575-16-4
- BV-IXδ
- δ-Biliverdin
|
|---|
|
Chemical Formula: |
C33H34N4O6 |
|---|
| Average Molecular Weight: |
582.66 |
|---|
| Monoisotopic Molecular
Weight: |
Not Available |
|---|
| InChI Key: |
INFDGUKTNKSIPV-JPDNPZEVSA-N |
|---|
| InChI: | InChI=1S/C33H34N4O6/c1-7-20-16(3)24(14-27-21(8-2)18(5)32(42)36-27)34-26(20)13-25-17(4)22(9-11-30(38)39)28(35-25)15-29-23(10-12-31(40)41)19(6)33(43)37-29/h7-8,13-15,34-35H,1-2,9-12H2,3-6H3,(H,36,42)(H,38,39)(H,40,41)/b25-13-,27-14-,28-15? |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 3-[(5Z)-2-[[3-(2-carboxyethyl)-4-methyl-5-oxopyrrol-2-yl]methylidene]-5-[[3-ethenyl-5-[(Z)-(3-ethenyl-4-methyl-5-oxopyrrol-2-ylidene)methyl]-4-methyl-1H-pyrrol-2-yl]methylidene]-4-methylpyrrol-3-yl]propanoic acid |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC1=C(C(=CC2=NC(=O)C(=C2CCC(=O)O)C)NC1=CC3=C(C(=C(N3)C=C4C(=C(C(=O)N4)C)C=C)C)C=C)CCC(=O)O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as tetrapyrroles and derivatives. These are polycyclic aromatic compounds containing four pyrrole rings joined by one-carbon units linking position 2 of one pyrrole ring to position 5 of the next. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Tetrapyrroles and derivatives |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Tetrapyrroles and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Tetrapyrrole skeleton
- Dipyrrin
- Dicarboxylic acid or derivatives
- Substituted pyrrole
- Pyrrole
- Pyrroline
- Heteroaromatic compound
- Carboxamide group
- Lactam
- Secondary carboxylic acid amide
- N-acylimine
- Carboxylic acid
- Azacycle
- Organic 1,3-dipolar compound
- Carboxylic acid derivative
- Propargyl-type 1,3-dipolar organic compound
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | Not Available |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
| Property | Value | Source |
|---|
| Molecular Weight |
582.657 g/mol | PubChem |
| XLogP3 |
2.5 | PubChem |
| Hydrogen Bond Donor Count |
5 | PubChem |
| Hydrogen Bond Acceptor Count |
7 | PubChem |
| Rotatable Bond Count |
11 | PubChem |
| Exact Mass |
582.248 g/mol | PubChem |
| Monoisotopic Mass |
582.248 g/mol | PubChem |
| Topological Polar Surface Area |
161 A^2 | PubChem |
| Heavy Atom Count |
43 | PubChem |
| Formal Charge |
0 | PubChem |
| Complexity |
1530 | PubChem |
| Isotope Atom Count |
0 | PubChem |
| Defined Atom Stereocenter Count |
0 | PubChem |
| Undefined Atom Stereocenter Count |
0 | PubChem |
| Defined Bond Stereocenter Count |
2 | PubChem |
| Undefined Bond Stereocenter Count |
1 | PubChem |
| Covalently-Bonded Unit Count |
1 | PubChem |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|