|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100066 |
|---|
|
Identification |
|---|
| Name: |
mupirocin |
|---|
| Description: | An α,β-unsaturated ester resulting from the formal condensation of the alcoholic hydroxy group of 9-hydroxynonanoic acid with the carboxy group of (2E)-4-[(2S)-tetrahydro-2H-pyran-2-yl]-3-methylbut-2-enoic acid in which the |
|---|
|
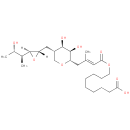
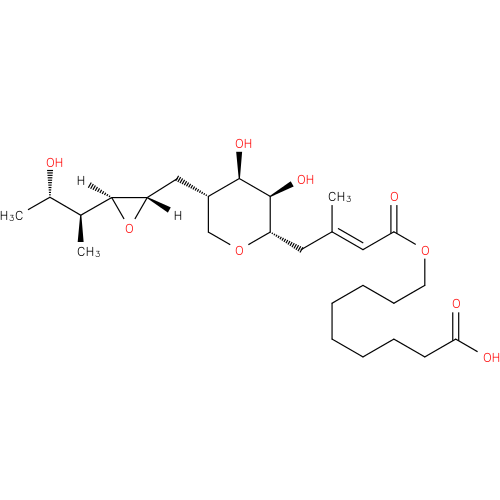
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C26H44O9 |
|---|
| Average Molecular Weight: |
500.6222 |
|---|
| Monoisotopic Molecular
Weight: |
500.299 |
|---|
| InChI Key: |
MINDHVHHQZYEEK-HBBNESRFSA-N |
|---|
| InChI: | InChI=1S/C26H44O9/c1-16(13-23(30)33-11-9-7-5-4-6-8-10-22(28)29)12-20-25(32)24(31)19(15-34-20)14-21-26(35-21)17(2)18(3)27/h13,17-21,24-27,31-32H,4-12,14-15H2,1-3H3,(H,28,29)/b16-13+/t17-,18-,19-,20-,21-,24+,25-,26-/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 9- ({(2E)- ({(2E)- 4- 4- [(2 [(2 |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | C[C@H](O)[C@H](C)[C@@H]1O[C@H]1C[C@H]1CO[C@@H](C\C(C)=C\C(=O)OCCCCCCCCC(O)=O)[C@H](O)[C@@H]1O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as medium-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 4 and 12 carbon atoms. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
|
Direct Parent |
Medium-chain fatty acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Medium-chain fatty acid
- Branched fatty acid
- Epoxy fatty acid
- Fatty acid ester
- Heterocyclic fatty acid
- Hydroxy fatty acid
- Dicarboxylic acid or derivatives
- Oxane
- Monosaccharide
- Enoate ester
- Alpha,beta-unsaturated carboxylic ester
- Carboxylic acid ester
- Secondary alcohol
- Ether
- Oxirane
- Dialkyl ether
- Carboxylic acid
- Organoheterocyclic compound
- Oxacycle
- Carboxylic acid derivative
- Carbonyl group
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
- monocarboxylic acid, secondary alcohol, epoxide, triol, alpha,beta-unsaturated carboxylic ester, oxanes (CHEBI:7025)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Melo JA, Ruvkun G. Inactivation of conserved C. elegans genes engages
pathogen- and xenobiotic-associated defenses. Cell. 2012 Apr 13;149(2):452-66. Pubmed: 22500807
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|