|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100062 |
|---|
|
Identification |
|---|
| Name: |
asphodelin A |
|---|
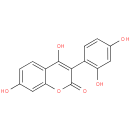
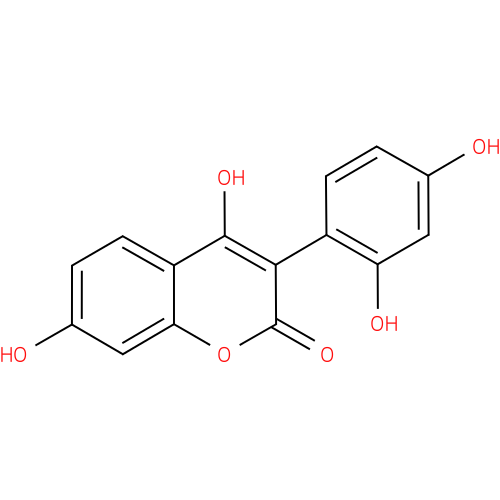
| Description: | A hydroxycoumarin that is 4,7-dihydroxy-2H-chromen-2-one substituted by a 2,4-dihydroxyphenyl group at position 3. It is isolated from the roots of Asphodelus microcarpus and exhibits antimicrobial activity against bacteria like Staphyloc |
|---|
|
Structure |
|
|---|
| Synonyms: | - 3-(2',4'-dihydroxyphenyl)-4,7-dihydroxy-2H-1-benzopyran-2-one
|
|---|
|
Chemical Formula: |
C15H10O6 |
|---|
| Average Molecular Weight: |
286.2363 |
|---|
| Monoisotopic Molecular
Weight: |
286.048 |
|---|
| InChI Key: |
OZOZCKVLUMXFGS-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C15H10O6/c16-7-1-3-9(11(18)5-7)13-14(19)10-4-2-8(17)6-12(10)21-15(13)20/h1-6,16-19H |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 3-(2,4-dihydroxyphenyl)-4,7-dihydroxy-2H-chromen-2-one |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | Oc1ccc(c(O)c1)-c1c(O)c2ccc(O)cc2oc1=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as hydroxyisoflavonoids. These are organic compounds containing an isoflavonoid skeleton carrying one or more hydroxyl groups. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
|
Class |
Isoflavonoids |
|---|
| Sub Class | Hydroxyisoflavonoids |
|---|
|
Direct Parent |
Hydroxyisoflavonoids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hydroxyisoflavonoid
- Isoflav-3-enone skeleton
- 4-hydroxycoumarin
- 7-hydroxycoumarin
- Hydroxycoumarin
- Coumarin
- Benzopyran
- 1-benzopyran
- Resorcinol
- 1-hydroxy-2-unsubstituted benzenoid
- 1-hydroxy-4-unsubstituted benzenoid
- Pyranone
- Phenol
- Benzenoid
- Pyran
- Monocyclic benzene moiety
- Vinylogous acid
- Heteroaromatic compound
- Lactone
- Oxacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
- polyphenol, hydroxycoumarin (CHEBI:65453)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- El-Seedi HR. Antimicrobial arylcoumarins from Asphodelus microcarpus. J Nat
Prod. 2007 Jan;70(1):118-20. Pubmed: 17253862
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
Not Available |
|---|