|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100059 |
|---|
|
Identification |
|---|
| Name: |
pyochelin |
|---|
| Description: | A member of the class of thiazolidines that is (4R)-3-methyl-1,3-thiazolidine-4-carboxylic acid which is substituted at position 2 by a (4R)-2-(2-hydroxyphenyl)-4,5-dihydro-1,3-thiazol-4-yl group. A siderophore, it is it is produced by Ps |
|---|
|
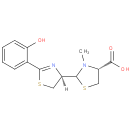
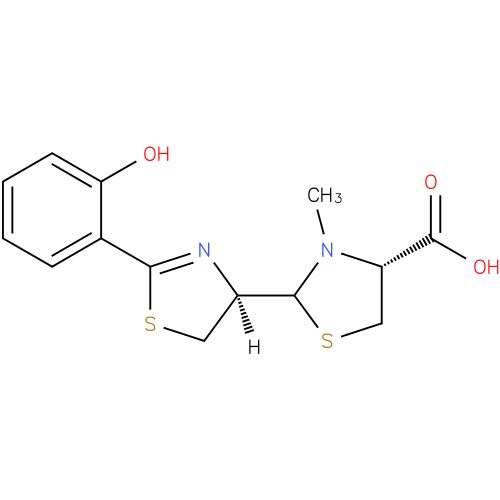
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C14H16N2O3S2 |
|---|
| Average Molecular Weight: |
324.418 |
|---|
| Monoisotopic Molecular
Weight: |
324.06 |
|---|
| InChI Key: |
NYBZAGXTZXPYND-GDVCOKDOSA-N |
|---|
| InChI: | InChI=1S/C14H16N2O3S2/c1-16-10(14(18)19)7-21-13(16)9-6-20-12(15-9)8-4-2-3-5-11(8)17/h2-5,9-10,13,15H,6-7H2,1H3,(H,18,19)/b12-8+/t9-,10+,13?/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (4R)-3-methyl-2-[(2E,4R)-2-(6-oxocyclohexa-2,4-dien-1-ylidene)-1,3-thiazolidin-4-yl]-1,3-thiazolidine-4-carboxylic acid |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | [H][C@@]1(CSC(=N1)c1ccccc1O)C1SC[C@H](N1C)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
L-alpha-amino acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- L-alpha-amino acid
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Imidothiolactone
- Thiazolidine
- Meta-thiazoline
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Thioether
- Hemithioaminal
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Dialkylthioether
- Azacycle
- Organoheterocyclic compound
- Organic oxide
- Carbonyl group
- Hydrocarbon derivative
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Organic oxygen compound
- Organopnictogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
- monocarboxylic acid, phenols, thiazolidines (CHEBI:29669)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Crosa JH, Walsh CT. Genetics and assembly line enzymology of siderophore
biosynthesis in bacteria. Microbiol Mol Biol Rev. 2002 Jun;66(2):223-49. Pubmed: 12040125
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|