|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100057 |
|---|
|
Identification |
|---|
| Name: |
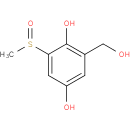
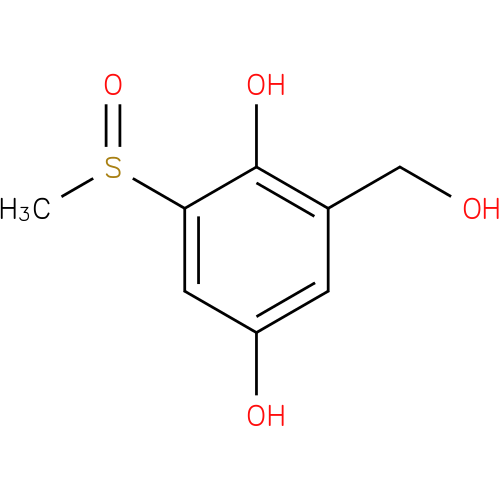
2,5-dihydroxy-3-methanesulfinylbenzyl alcohol |
|---|
| Description: | A benzyl alcohol with hydroxy substituents at positions 2 and 5 and a methanesulfinyl group at position 3. It is a fungal secondary metabolite from Ampelomyces sp. SC0307 and has antibacterial activity against Escherichia coli, Pseudomona |
|---|
|
Structure |
|
|---|
| Synonyms: | Not Available |
|---|
|
Chemical Formula: |
C8H10O4S |
|---|
| Average Molecular Weight: |
202.228 |
|---|
| Monoisotopic Molecular
Weight: |
202.03 |
|---|
| InChI Key: |
NADRVFUNXRGAII-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C8H10O4S/c1-13(12)7-3-6(10)2-5(4-9)8(7)11/h2-3,9-11H,4H2,1H3 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 2-(hydroxymethyl)-6-(methylsulfinyl)benzene-1,4-diol |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CS(=O)c1cc(O)cc(CO)c1O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as phenyl sulfoxides. These are organosulfur compounds containing a sulfoxide group substituted with a phenyl group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
|
Class |
Benzene and substituted derivatives |
|---|
| Sub Class | Phenyl sulfoxides |
|---|
|
Direct Parent |
Phenyl sulfoxides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Phenyl sulfoxide
- Benzyl alcohol
- Hydroquinone
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Sulfoxide
- Sulfinyl compound
- Alcohol
- Aromatic alcohol
- Primary alcohol
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
- benzyl alcohols, sulfoxide, hydroquinones (CHEBI:65779)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Zhang H, Xie H, Qiu SX, Xue J, Wei X. Heteroatom-containing antibacterial
phenolic metabolites from a terrestrial Ampelomyces fungus. Biosci Biotechnol
Biochem. 2008 Jul;72(7):1746-9. Pubmed: 18603789
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
Not Available |
|---|