|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100049 |
|---|
|
Identification |
|---|
| Name: |
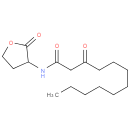
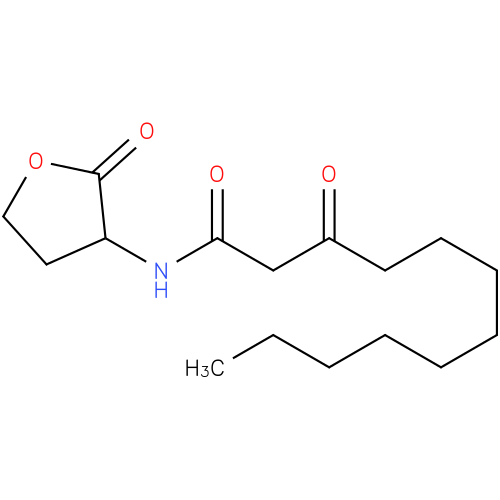
N-(3-oxododecanoyl)homoserine lactone |
|---|
| Description: | A N-acyl homoserine lactone that is the monocarboxylic acid amide arising from formal condensation of homoserine lactone with 3-oxododecanoic acid. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 3-oxo-C12-AHL
- 3-oxo-C12-HSL
- 3-Oxo-N-(tetrahydro-2-oxo-3-furanyl)dodecanamide
- N-(3-ketododecanoyl)homoserine lactone
- N-(3-Oxododecanoyl)homoserine lactone
- N-(3-oxododecanoyl)homoserine lactone
|
|---|
|
Chemical Formula: |
C16H27NO4 |
|---|
| Average Molecular Weight: |
297.3899 |
|---|
| Monoisotopic Molecular
Weight: |
297.194 |
|---|
| InChI Key: |
PHSRRHGYXQCRPU-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C16H27NO4/c1-2-3-4-5-6-7-8-9-13(18)12-15(19)17-14-10-11-21-16(14)20/h14H,2-12H2,1H3,(H,17,19) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 3-oxo-N-(2-oxotetrahydrofuran-3-yl)dodecanamide |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CCCCCCCCCC(=O)CC(=O)NC1CCOC1=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as n-acyl-alpha amino acids and derivatives. These are compounds containing an alpha amino acid (or a derivative thereof) which bears an acyl group at its terminal nitrogen atom. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
N-acyl-alpha amino acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-amino acid ester
- N-acyl-alpha amino acid or derivatives
- Acyl-homoserine lactone
- Beta-hydroxy ketone
- Gamma butyrolactone
- Tetrahydrofuran
- Carboxylic acid ester
- Ketone
- Lactone
- Carboximidic acid
- Carboximidic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organopnictogen compound
- Carbonyl group
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
- N-acyl homoserine lactone (CHEBI:29639)
- Fatty acyl homoserine lactones (C11840)
- an acyl-homoserine lactone (CPD-10783)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Debler EW, Kaufmann GF, Kirchdoerfer RN, Mee JM, Janda KD, Wilson IA. Crystal
structures of a quorum-quenching antibody. J Mol Biol. 2007 May
18;368(5):1392-402. Epub 2007 Mar 6. Pubmed: 17400249
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| KEGG COMPOUND | C11840 |
|
|---|