|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100038 |
|---|
|
Identification |
|---|
| Name: |
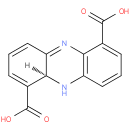
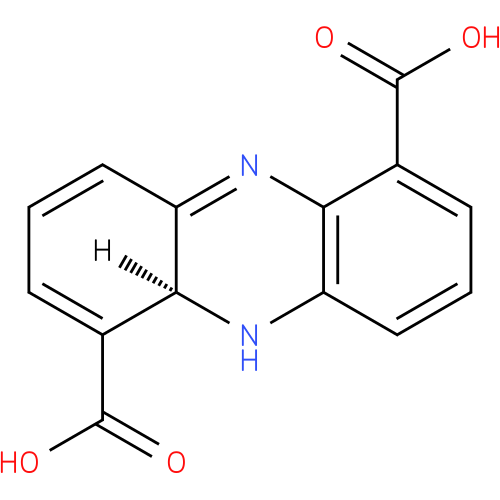
(5aS)-5,5a-dihydrophenazine-1,6-dicarboxylic acid |
|---|
| Description: | A member of the class of phenazines that is (5aS)-5,5a-dihydrophenazine substituted at positions 1 and 6 by carboxy groups. |
|---|
|
Structure |
|
|---|
| Synonyms: | Not Available |
|---|
|
Chemical Formula: |
C14H10N2O4 |
|---|
| Average Molecular Weight: |
270.241 |
|---|
| Monoisotopic Molecular
Weight: |
270.064 |
|---|
| InChI Key: |
INPMVLIHPFWVLB-NSHDSACASA-N |
|---|
| InChI: | InChI=1S/C14H10N2O4/c17-13(18)7-3-1-5-9-11(7)16-10-6-2-4-8(14(19)20)12(10)15-9/h1-6,11,16H,(H,17,18)(H,19,20)/p-2/t11-/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (5aS)-5,5a-dihydrophenazine-1,6-dicarboxylic acid |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | C1=CC=C([C@]2(C1=NC=3C(=CC=CC3N2)C(=O)O)[H])C(=O)O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as phenazines and derivatives. These are polycyclic aromatic compounds containing a phenazine moiety, which is a linear tricyclic system that consists of a two benzene rings joined by a pyrazine ring. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Diazanaphthalenes |
|---|
| Sub Class | Benzodiazines |
|---|
|
Direct Parent |
Phenazines and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Phenazine
- Secondary aliphatic/aromatic amine
- Dicarboxylic acid or derivatives
- Benzenoid
- Amino acid
- Ketimine
- Amino acid or derivatives
- Carboxylic acid derivative
- Carboxylic acid
- Secondary amine
- Azacycle
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Imine
- Amine
- Organic nitrogen compound
- Organic oxygen compound
- Carbonyl group
- Organopnictogen compound
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Xu N, Ahuja EG, Janning P, Mavrodi DV, Thomashow LS, Blankenfeldt W. Trapped
intermediates in crystals of the FMN-dependent oxidase PhzG provide insight into
the final steps of phenazine biosynthesis. Acta Crystallogr D Biol Crystallogr.
2013 Aug;69(Pt 8):1403-13. Pubmed: 23897464
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|