|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100034 |
|---|
|
Identification |
|---|
| Name: |
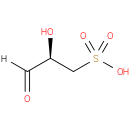
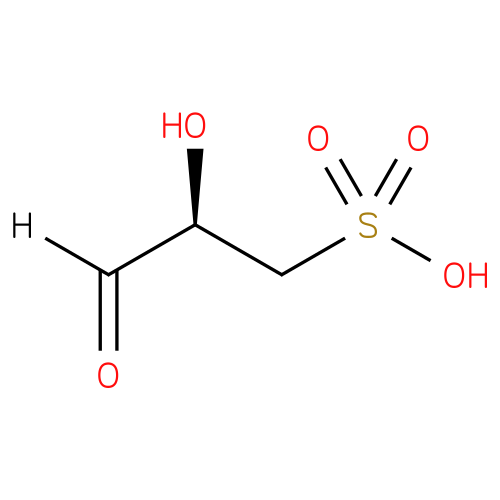
D-3-sulfolactaldehyde |
|---|
| Description: | A 3-sulfolactaldehyde in which the stereocentre at position 3 has R-configuration. |
|---|
|
Structure |
|
|---|
| Synonyms: | Not Available |
|---|
|
Chemical Formula: |
C3H6O5S |
|---|
| Average Molecular Weight: |
154.143 |
|---|
| Monoisotopic Molecular
Weight: |
153.994 |
|---|
| InChI Key: |
GVEIZEMJOBQMCQ-GSVOUGTGSA-N |
|---|
| InChI: | InChI=1S/C3H6O5S/c4-1-3(5)2-9(6,7)8/h1,3,5H,2H2,(H,6,7,8)/t3-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (2R)-2-hydroxy-3-oxopropane-1-sulfonic acid |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | OS(C[C@@H](C(=O)[H])O)(=O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as organosulfonic acids. These are compounds containing the sulfonic acid group, which has the general structure RS(=O)2OH (R is not a hydrogen atom). |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Organic sulfonic acids and derivatives |
|---|
| Sub Class | Organosulfonic acids and derivatives |
|---|
|
Direct Parent |
Organosulfonic acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-hydroxyaldehyde
- Alkanesulfonic acid
- Sulfonyl
- Organosulfonic acid
- Secondary alcohol
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Carbonyl group
- Aldehyde
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Felux AK, Spiteller D, Klebensberger J, Schleheck D. Entner-Doudoroff pathway
for sulfoquinovose degradation in Pseudomonas putida SQ1. Proc Natl Acad Sci U S
A. 2015 Aug 4;112(31):E4298-305. Jul 20. Pubmed: 26195800
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
Not Available |
|---|